* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Gastric hormones handout text
Survey
Document related concepts
Transcript
ENDOCRINOLOGY 7 GASTRO-INTESTINAL HORMONES
Coordination of G-I tract Function
Functions:
• G-I hormones, with the enteric and autonomic nervous systems, integrate and coordinate the
mechanisms which move, digest and absorb the various meals that are ingested, at the same
time preserving homeostasis of the milieu interne.
• They control G-I tract exocrine and endocrine secretion, motility, growth, & blood flow.
• They also affect appetite and feeding behaviour
Routes:
• Hormones released from gut endocrine cells act via endocrine and paracrine routes (and
possibly also via the gut lumen).
• Peptides are also released from nerves of the enteric nervous system.
• Because many actions are local and not via the blood stream it is difficult to determine the
physiologically active concentration of hormone. Antibodies which neutralise the action of gut
hormones have therefore been used to elucidate their physiology.
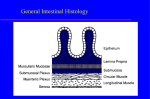
Gut (entero-) endocrine cells:
• Part of G-I tract epithelium; derived from endoderm; positioned in crypts;
• Most cells have sensory microvilli on apex open to gut lumen;
• Hormone is secreted basally;
• Distribution varies fetus/adult (e.g. in fetus most gastrin is in pancreas).
Gut hormones:
• Evolution has produced families: gastrin/CCK; secretin/VIP/GIP/glucagon; (many have
homologues in the brain);
• Most are peptides produced from larger precursors, so different molecular forms of the
hormones are found;
• An endocrine role is established for most, others are candidate hormones;Many show Amine
Precursor Uptake & Decarboxylation (APUD cells; hence clinically tumours are sometimes called
“APUDomas”).
GASTRIN –
stimulates gastric acid
& pepsinogen
secretion and motility
• Distribution: G cells
of gastric antrum
crypts; some duodenal.
• Synthesis:
preprogastrin →
gastrin G34 → G17 →
G13 + G4
(pentagastrin)(sulphati
on)
Release of gastrin after meal
1
• Plasma: fasting 10-50pmol/l; increased by 40 pmol/l after meal; G17 t0.5=5 min; (G34
t0.5=40 min has only 20% potency of G17)
• Mechanism of action: via Gαq, Ca, PKC
• Release:
Stimulated by:
protein digestion products (esp tryptophan,
phenylalanine)(also beer, wine, coffee)
vagus, via acetylcholine, gastrin-releasing peptide
distension of stomach
hypercalcaemia (cause?
)
Inhibited by:
stomach pH<2.5; alkali short-term has little effect, long-term
causes hyperplasia;
somatostatin (local negative feedback)
Trophic effects of gastrin
• Actions:
stimulates gastric acid secretion (direct & indirect via histamine H2); stimulates parietal
cell growth
stimulates pepsinogen secretion from chief cells; (also water &
electrolyte secretion in liver, pancreas, intestine)
stimulates antral motility, mucosal blood flow
trophic to body of stomach; ? to other parts
(high levels: stimulate calcitonin, food intake, insulin secretion,
pancreatic enzyme secretion, antagonise secretin)
• Pathology: gastrinoma (usually in pancreas) causes repeated peptic
ulceration due to high acid and pepsin secretion
NB Effect on acid secretion synergises with that of vagal acetylcholine
(M3 receptors Gαq, Ca, PKC)
Effects of histamine and H2 antagonists
HISTAMINE – stimulates gastric acid secretion
• Distribution: Enterochromaffin-like (ECL) cells of stomach wall.
• Synthesis: from histidine by histidine decarboxylase
• Release: Stimulated by vagus (ACh), gastrin
• Actions: Stimulates gastric acid (HCl) secretion via H2 receptors
(Gαs, cAMP, PKA)
• Pharmacology: H2 receptor antagonists used to treat peptic
ulceration (now largely superceded by proton (H-K) pump
inhibitors)
2
GASTRIN-RELEASING PEPTIDE
peptide; in nerves of GI tract (vagus)(also lung mucosal
neuroendocrine cells); releases gastrin
SECRETIN – neutralises stomach acid
• Distribution: S cells, duodenum to distal ileum; in neck of crypts
• Synthesis: small, very basic peptide. Plasma: 20pmol/l after meals; t0.5=3 min
• Release: stimulated by acid in proximal duodenal lumen (pH<4.5);
somatostatin
inhibited by
• Actions: via cAMP
- stimulates pancreatic ducts to secrete HCO3- & water; (cAMP stimulates Cl- secretion
via CFTR, HCO3- is then exchanged for Cl-) this washes pancreatic enzymes into the gut.
- stimulates liver secretion of HCO3- & water into bile
(potentiates CCK)
NB synergises with vagal acetylcholine (M3 receptors Gαq, Ca, PKC)
CHOLECYSTOKININ (CCK; aka PANCREOZYMIN) - secretion of pancreatic enzymes and bile into
duodenum
• Distribution: I cells in the duodenum and jejunum
• Forms: CCK33, CCK58, CCK39; CCK8 (in brain); shares terminal pentapeptide with gastrin
• Release:
stimulated by protein and fat digestion products in duodenum
3
• Actions: (via Gαq, Ca, PKC)
- stimulates secretion of pancreatic enzymes and potentiates action of secretin
- stimulates contraction of gall bladder
(inhibits gastric emptying; increases small bowel transit)
(high levels potentiate secretion of calcitonin)
- causes satiety (feeling one has had enough to eat) via vagal nerve terminals and in the
CNS
NB synergises with vagal ACh (M3 receptors Gαq, Ca, PKC)
THE ‘INCRETIN’ EFFECT - glucose stimulates insulin more powerfully if
than intravenously
GLP-1[7-37] Glucagon-like peptide (a type of gut glucagon)
• Distribution: L cells in small bowel (also produce oxyntomodulin – see below)
• Release: stimulated by meals: especially oral (not intravenous)
fat; also by GIP
• Actions: powerfully potentiates glucose-stimulated insulin release
effect); alone, does not stimulate insulin
GIP (Glucose-dependent Insulinotropic Peptide) (Originally named for its
gastric inhibitory peptide action, which is weak)
• Distribution: K cells of duodenum > proximal jejunum > distal jejunum
• Structure:
43aa peptide of secretin/glucagon family
given orally
carbohydrate and
("incretin"
• Release:
stimulated by oral (not intravenous) glucose, fat and protein;
insulin may inhibit release
• Actions: potentiates glucose-stimulated insulin release ("incretin"
effect); alone,
does not stimulate insulin
LOCAL INHIBITION/NEGATIVE FEEDBACK
SOMATOSTATIN
• Distribution: D cells from gastric antrum through to colon
• Structure:
small (14aa) peptide (28aa in brain); acts via inhibition of cAMP production
• Release: stimulated by meals (amino acids, glucose, fatty acids), gastrin,
• Actions: suppresses secretion of most GI hormones and their effects
paracrine negative feedback
retards absorption of glucose and so protects against
hyperglycaemia (in liver)
secretin
(acid etc);
post-prandial
• Pathology: Tumour - somatostatinoma.
Effects: steatorrhoea (fatty faeces; lack of CCK);
hypoacid (lack of gastrin); bowel stasis (lack of motilin).
diabetes mellitus (lack of insulin);
• Pharmacology: somatostatin analogue (eg Octreotide; 8aa) used to treat gut endocrine
tumours; also to treat acromegaly.
4
PANCREATIC POLYPEPTIDE
• Distribution: Islets in uncinate process of (ventral) pancreas
• Structure: small peptide related to neuropeptide Y; Y4 receptor, inhibition of cAMP
• Release: stimulated by vagus, nutrients (not glucose) gastric distension,
inhibited by hyperglycaemia, somatostatin
• Actions: inhibits postprandial pancreatic enzyme secretion
reduces gastric and intestinal motility
reduces appetite centrally
• Pathology: secreted by some GI tumours, used as tumour marker.
Other major gut hormones
Vasoactive Intestinal Polypeptide - VIP
• Distribution: in enteric neurons (also brain; and in pelvic parasympathetic nerves where, with
NO, it is causes penile erection)
• Structure:
small peptide; secretin/glucagon family; acts via cAMP (c.f. cholera)
• Release:
?oesophageal distension; plasma levels don't rise after meal
• Actions:
relaxes cardiac sphincter and stomach, anal sphincter; vasodilator
Pathology: Tumour - VIPoma (Verner-Morrison syndrome): watery diarrhoea; hypokalaemia;
achlorhydria
Motilin
• Distribution:
gut endocrine cells from gastric antrum to colon
• Structure: small peptide
• Actions:
increases the motility of the bowel; induces migrating myoelectric
complexes at antro-duodenal pacemaker
Neurotensin
• Distribution:
N cells of distal small intestine (also extensively in CNS neurons)
• Structure: small peptide
• Stimuli:
intraluminal fat
• Actions:
physiology unknown: reported to pancreatic secretion; gastric/ small bowel
motility; colonic motility
trophic effects on gut mucosa
Gut glucagon (enteroglucagon, glicentin)
• Distribution:
Gastric A cells; ileal and colonic L cells
• Structure: extended forms of glucagon-like molecules
• Actions:
stimulates gastric/intestinal motility; intestinal absorption sugars; has systemic
glucagon-like effects
trophic to small bowel (compensatory hypertrophy)
5
Pathology: Multiple Endocrine Neoplasia (MEN)
Tumours are usually multiple; both types are genetically determined)
MEN 1: tumours of pancreas (gastrinoma, insulinoma, VIPoma), pituitary and
MEN 2: tumours of thyroid C cells, adrenal chromaffin cells and parathyroids
GUT HORMONES IN THE CONTROL OF APPETITE AND FEEDING
In addition to the feedback effects from taste, stomach distension etc by the sensory nerves of the
alimentary tract, it is now clear that gastrointestinal hormones affect feeding by an action on the
hypothalamus and brain stem (via the blood). The same is true for insulin, which suppresses
appetite. This is dealt with more fully in year 2 integrative physiology
CCK – inhibits feeding (see above)
PANCREATIC POLYPEPTIDE – inhibits feeding (see above)
GHRELIN – stimulates feeding
Distribution: produced by stomach parietal cells in fasting conditions.. Levels rise 1-2h before a
meal and decline to a minimum afterwards
Structure: small peptide
Actions: powerful stimulator of feeding by action in hypothalamus and brain stem
Pathology: increased in people who have lost weight by dieting. Plasma ghrelin is very high in
(rare) Prader-Willi obesity
PEPTIDE YY (PYY) – inhibits feeding
Distribution: produced in ileum and more distal bowel after meals
Structure: small peptide
Actions: has a prolonged action on Y2 receptors in hypothalamus to decrease food intake.
STOP PRESS
Two other hormones – oxyntomodulin (produced in small intestine from the same gene as
glucagon) and obestatin (very recently discovered in the stomach and derived from same gene as
ghrelin) are now being actively researched, with pancreatic polypeptide, as appetite suppressants.
OTHER SITES OF HORMONE PRODUCTION
Respiratory tract endocrine cells
There are similar endocrine cells in the epithelium of the lower respiratory tract
Adipose tissue – dealt with next year
LEPTIN: peptide produce by adipocytes in relation to amount of body fat; inhibits appetite by
action in hypothalamus
ADIPONECTIN: peptide acting in muscle and liver to increase sensitivity to insulin. Decreased
plasma adiponectin is associated with the ‘metabolic syndrome’ of increased BMI,
insulin resistance and plasma lipid disturbance.
Heart
ATRIAL NATRIURETIC PEPTIDE
Production: small peptide secreted by atrial myocytes
6
Release:
atrial dilatation (i.e. increased venous return, right heart failure)
Actions: stimulates loss of sodium (and water) in urine probably by an effect on glomeruli
inhibits renin-angiotensin-aldosterone system
reduces blood pressure (reduces venous return and cardiac output)
Kidneys
ERYTHROPOIETIN
Production: glycoprotein produced by epithelial cells of glomeruli (and liver)
Release: stimulated by reduced oxygen saturation of the blood (HIF); also by androgens and βadrenergic effects
Actions: stimulates production of erythrocytes in bone marrow
Abuse: by athletes wishing to improve the oxygen-carrying capacity of their blood. The risk is that
the blood will become too viscous.
RENIN-ANGIOTENSIN SYSTEM
Renin - an acid protease produced by juxtaglomerular cells in the afferent arterioles of renal
glomeruli
Release: stimulated by sodium depletion, hypotension, dehydration, low renal artery blood flow,
sympathetic nerve stimulation
Renin cleaves plasma angiotensinogen to angiotensin I which is then cleaved by lung angiotensinconverting enzyme (ACE) to active angiotensin II, which stimulates aldosterone secretion, thirst,
and vasoconstriction.
Pharmacology: ACE inhibitors used in the treatment of hypertension.
Renal 1α-hydroxylase activates 25(OH)D3 to 1,25(OH)2D3 (see bone lecture; calcium control
lecture in Y2 Integrative Physiology).
General
Most tissues produce PROSTANOID/EICOSANOID hormones (prostaglandins, leukotrienes,
thromboxanes). These act locally to a large extent (e.g. in inflammation) but can act at a distance –
contraction of uterus in childbirth; effects on ductus arteriosus. Remember that some
prostaglandins (PGF2, PGE2) stimulate the contraction of smooth muscle (e.g. in uterine
contraction at parturition) whereas others (PGI = prostacyclin) inhibit the contraction (e.g. in
vasodilation)
Gonads, placenta
Hormones produced by the gonads and placenta were dealt with in the Reproduction lectures.
7