* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Monocots vs
Plant use of endophytic fungi in defense wikipedia , lookup
Photosynthesis wikipedia , lookup
Plant stress measurement wikipedia , lookup
History of botany wikipedia , lookup
Plant defense against herbivory wikipedia , lookup
Evolutionary history of plants wikipedia , lookup
Plant nutrition wikipedia , lookup
Plant breeding wikipedia , lookup
Plant secondary metabolism wikipedia , lookup
Plant ecology wikipedia , lookup
Plant physiology wikipedia , lookup
Plant reproduction wikipedia , lookup
Plant morphology wikipedia , lookup
Sustainable landscaping wikipedia , lookup
Perovskia atriplicifolia wikipedia , lookup
Plant evolutionary developmental biology wikipedia , lookup
Monocotyledon wikipedia , lookup
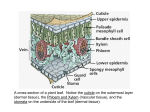
Nick Lorenz IB 423 Final 2. The plant kingdom has not always been divided up into two clear cut families such as monocots and dicots. In fact, today the distinction is not prefect as well. Yet today we use the distinction of these two groups to facilitate describing plant species that are studied because there are a large number of differences between the groups and they are fairly consistent across taxa. From the acknowledgement of their differences, we can then ask about the evolution of these two groups. Did they both branch off from a common ancestor or is one derived from the other? The relationship seems to be the later case. Current hypotheses about the origin and diversification of the flowering plants suggest that the dicots are the older group, from which the monocots evolved. In fact, some dicots, called paleoherbs, are now believed to be closer relatives of the monocots than of the other dicots. In other words, the dicots include a basal paraphyletic group from which the monocots evolved. It is now believed that some of the dicots are more closely related to monocots than to the other dicots, and that the angiosperms do not all fit neatly into two clades. Counting species, there are at least three times more dicots than monocots (60,000 species). With the assistance of evidence from DNA sequences, now scientists with confidence place monocots as a branch within the dicotyledons, further destroying the old model that depicts two branches at the base. So how did they actual decide monocots were more advanced than dicots, and was this possible even before DNA sequencing? It seems that the two groups have different strategies and both make sense. In general dicots have more specialization in tissue development and are also more organized to a specific plant structure. This strategy argues for the best consistency among species, and if the structure is successful then you want to make it consistent. On the other hand monocots have a simpler structure. There is less tissue specialization because some of the same tissues in dicots take on other tasks which negate the necessity of a specialized tissue (the lack of periderm will depict this). There is also a reduction in plant parts for monocots. While this may seem primitive at first, it also may tell of the increased efficiency in monocots. The randomness of some parts of monocots may actually point towards this hypothesis as well. Starting off with the most apparent difference is the number of cotyledons. Monocots have one less cotyledon in their embryo than dicots obviously, and this tells us about the differences is quality. The single cotyledon has everything the plant needs and it is able to have primary development from one source. The dicot plant may be using up more resources in creating that second cotyledon thus making it less efficient than the monocot. In the leaf venation the monocot again seems simpler than the dicot leaf. Dicots have palmate major vein formation and reticulate minor veins that are immersed in the mesophyll and make sure every cell is at least in the vicinity of a nutrient supplying vein. The dicot venation pattern looks very complex. The parallel venation of monocot leaves looks less appealing but if you think about its structure it seems like it would provide the plant with a better system for supplying nutrients to leaves. Since the pattern does not branch the nutrient flow is very direct and fast, straight from the main body of the plant. Dicots do not do this because the branching allows for the minor veins to reach every cell. However the monocot does this as well with commissural veins that connect parallel major veins. In fact, this system seems better because it is less random and all cells seem to be more equal in their nutrient intake. The reticulate pattern of dicot may put some cells at a greater distance from the nutrient source. Monocots have an atactostele also are more random in their vascular bundles than dicots and this may have the same strategy as their leaf venation. Dicots instead have a ring of vascular bundles in their subepidermal tissues. Both of these systems obviously work very well, but the monocot random development shows that vascular bundles develop where they are most needed. This feature is not unique to monocots, but is found also in the paleoherbs and certain magnolia-like dicots. Vascular tissue like xylem not only provides nutrients but also provides support and when the xylem develops randomly it develops where it is necessary. Using the word random seems to imply there is no purpose, but that would be wrong. It seems just the opposite, that vascular bundles develop in plants were they are sensed to be needed the most. This may insure that all cells receive a more equal amount of water from xylem and nutrients from phloem. The transfer of water and nutrients within dicots is probably much slower for cells that are far from the subepidermal ring. Since monocots do not have a continuous vascular cambium they do not have secondary growth, which initially sounds like a disadvantage. However, monocots develop with adequate vascular systems so they seem more efficient. In fact, they probably waste less primary tissue that is frequent in dicots after their secondary development. Like having no vascular cambium, monocots have no periderm, but it seems unnecessary to have separate tissues to achieve the periderm function. In monocots they inside have the outer epidermis become thickened, suberized and/or sclerified. This outer protective layer is even more specialized than a dicot periderm. Within the monocot outer cortex, parenchyma cells become meristematic and through periclinal divisions they produce short radial files (similar to phellem). These are non-living and heavily suberized. Some non-suberized cells become trapped between suberized zones but they receive the same protection with the need for more suberin production. This process seems much more efficient than the periderm in dicots (Beck 246). Another feature common to all monocots is the structure of certain plastid inclusions. All plant cells contain plastids, some of which become photosynthetic, but those which are found in the phloem tissue do not do so. When examined in properly prepared material, the plastids in the phloem are shown to contain crystalloid proteins, the shape of which is distinctive for different major groups of plants. With an overall abundance of chloroplasts, the monocot group is able to perform photosynthesis more often if necessary, which is great for peak photosynthetic periods. While most monocots are herbs, and lack vascular cambium and true wood, they do have the ability to create similar forms. At least four times in their history, monocots have evolved tree-like growth forms. A lack of cambium might be expected to make the evolution of tree-like growth forms difficult, but it obviously has not been an insurmountable obstacle. In monocots species like palms, grass-trees, bamboo, pandanes, and yuccas all simulate woody growth. DNA analysis has even been used to show that monocots are evolved from dicots. The most useful multigene analysis so far has been based on nucleotide sequences of three genes, rbcL and atpB from chloroplasts and 18s for ribosomal DNA sequences from the nucleus. By analyzing the patterns of evolution for all three genes at the same time, a computer produced an evolutionary tree having a very high degree of support. From this analysis, one conclusion was inescapable: the monocots evolved from dicotyledonous stock, not from the base of the tree, and monocots originated after several groups of dicotyledons had already evolved. Computer analyses tell us that we can be essentially 100% certain that ALL monocots arose as a single evolutionary lineage (monophyletic). Theoretically this means that all 2800 monocot genera on earth today can trace their origins back to a single ancestral population. Thus probably ends another age-old debate, whether monocots evolved more than one time. 3. After an ovule is fertilized it begins to expand and develops into a seed The ovary eventually comes to form, along with other parts of the flower, a structure surrounding the seed called the fruit. Fruit development continues until the seeds have matured. We see a wide variety of fruits and this is a result of the differences in the number of ovules that are fertilized. Fruits are so varied in form and development, that it is difficult to devise a classification scheme that includes all known fruits. There are three different types of fruits: simple, aggregate, and multiple. What type of fruit develops is thought to be controlled by the phase of rapid cell division. The number of fertilized ovules in a fruit is correlated with both the initial cell division rate and the final size of the fruit. Simple fruits can be either dry or fleshy and consists of a single carpel, or several fused carpels, without any attached floral parts. Dry fruits may be either dehiscent or indehiscent. This is the most abundant groups with many different groups each with a multitude of species. There dry fruits like achenes (buttercup), caryopsis (wheat), fiberous drupe (coconut, walnut), legume (pea, bean, peanut) and many more. Fleshy simple fruits are berry (tomato, avocado), drupe (plum, cherry, peach) and others. These simple fruits develop from hypogynous flowers in which the ovaries are superior. An aggregate fruit is one that consists of several to many carpels of a single flower. An example is the raspberry, whose simple fruits are termed drupelets because each is like a small drupe attached to the receptacle. In a bramble fruit, like blackberry the receptacle is elongate and part of the ripe fruit, making the blackberry an aggregateaccessory fruit. The strawberry is also an aggregate-accessory fruit, but the seeds are contained in achenes. In all these examples, the fruit develops from a single flower with numerous carpels (Beck 370). Multiple fruits consists of the fused ovaries of several flowers (called an inflorescence). Each flower produces a fruit, but these mature into a single mass. First an inflorescence of flowers called a head is produced. After fertilization, each flower develops into a drupe, and as the drupes expand, they merge into a multiple fleshy fruit called a syncarp. Examples are the pineapple, edible fig, mulberry, osage-orange, and breadfruit. If these fruits are composed of floral parts other than the carpels they are termed accessory fruits. 6. Plastids can have many functions and are responsible for photosynthesis, storage of products like starch and for the synthesis of many classes of molecules such as fatty acids, which are needed as cellular building blocks and/or for the function of the plant. Depending on their morphology and function, plastids are commonly classified as chloroplasts, leucoplasts, amyloplasts or chromoplasts. However, these different forms are not fixed, and plastids have the ability to differentiate, or redifferentiate, between these forms. All plastids are derived from proplastids, which are present in the meristematic regions of the plant. Proplastids and young chloroplasts commonly divide, but more mature chloroplasts also have this capacity. Undifferentiated plastids (proplastids) will differentiate into several forms, depending upon which function they need to play in the cell. The most commonly known plastid is chloroplasts which contain chlorophyll along with photosystems II and I for photosynthesis. Photosynthesis results in primary assimilate starch. Closely related to chloroplasts would be amyloplasts because they contain thylakoids and are vital to starch accumulation also. However, amyloplasts occur in non-green cells like roots and seeds and are more important to long-term or secondary starch storage. Both plastids accumulate starch in the stroma. Similar to amyloplasts (but lacking starch) are eliaoplasts. They store different materials instead like oils and fatty acids for the plant which could be later secreted or consumed. Leucoplasts have a little in common with the two previous plastids, but the starch accumulation is much less common and they also lack pigmentation and ribosomes in mature form. They act like continual proplastids and keep replicating in new dividing cells so that they could develop into other plastid forms. Chromoplasts are important to pigment synthesis and storage. They have very large plastoglobuli which store the carotenoids. Since they lack a thylakoid system and starch grains, chromoplasts see to be less related to other plastids. Etioplasts are like chloroplasts which have not been exposed to light. Instead of a large thylakoid system they have a lattice of tubules called prolamellar bodies. Their growth is very different to light sufficient cells as their vertical growth is accelerated but their leaves are usually white or yellow. What makes this occur is their inhibition of chlorophyll production by protochlorophyllide. When introduced back to the sun, the light will degrade protochlorophyllide and chlorophyll accumulates, causing the thylakoid system to develop. An etioplast can morph into a chloroplast within minutes. The development and specialization of plastids are enough to hint that they have had a rich evolution in order to have such complexity and diversity. However it was not always that plants developed their plastids from their own DNA (and it still really is not the case). A process better understood now is prokaryotic genome assimilation into the plant nucleus. This arose, way back in prehistoric time, when plant ancestors assimilated photosynthetic prokaryotes. These unicellular organisms, like bacteria, lacked a membrane bound nucleus unliked eukaryotes. For the creation of functional plastids in plants there needed to be migration of huge numbers of genes from the prokaryote genome to the plant nucleus. Scientists have a relatively good idea about the identity and gene content of the organisms that were involved when the original eukaryotic plant ancestor took in the prokaryotic forerunner of the plastid. Currents research is trying to figure out the evolutionary process of gene transfer from the plastid to the nucleus and also ask is transfer of plastid DNA into the nucleus of higher plants still occurring? How did scientists make this discovery? After analyzing plastid DNA and completing the sequence for the genome, they were able to deduce that the genome has clear remanents of eubacterial genes. By comparing gene sequence, organization, and expression of plastids with similar data on varying eubacteria, researchers were able to pinpoint the evolutionary origin of plastids (along with mitochondria) and identify extant eubacterial lineages to which they are closely related (Gray 21). While they were not able to define a specific lineage, scientists decided that plastids orginated from within Cyanobacteria. A discovering this origin of plastids some scientists then went on to finding out how many times did plastids orgininate in evolution? Also is their any non-eubacterial proteins in plastids and where did they come from and when and how? The current plastid genome helped them answer some of these questions. Plastid genomes are pretty large (100-200kb) compared to say a mitochondrial genome. They also have a lot of genes but they are much less diverse in structure and they tend to be conservative in their retention of ancestral eubacterial features. Scientists have looked at this conservation and used it as evidence to support the hypothesis of a monophyletic evolution of plastids. There are some who still argue for a polyphyletic evolution, but there is no strong evidence to support this alternate hypothesis. The initial assimilation of plastids is thought to have occurred by primary endosymbiosis in which cyanobacteria was taken up by a phagotrophic eukaryotic host. Plastids that derived from this are called primary plastids and are in three eukaryotic lineages: red algae, green algae, and glaucophytes. However, it became very difficult to unite a host component for these three types of algae in phylogeny. This could point to a very early acquisition of a primary plastid and a following diversification and plastid loss in some eukaryotic lineages (which are now aplastidic). It has also been found that some algae have obtained plastids via secondary endosymbiosis. In this process they took up a another photosynthetic alga already containing a plastid. This addition of plastids via horizontal transfer is important to the abundence and advancement of plastids. However they have decided that secondary plastids must have derived independantly at least twice because we have green and red algae. When looking at the evolution of plastids into land plants, they have noticed that a lot of rRNA is conserved (81%) from land plants to chlorophyte algae. This conservation of genes allow scientists to divide the plastid genome into two repeat and two single-copy regions. Most of the conservation is within certain regions and in clusters, reinforcing the hypothesis of extreme conservation of the genome amongst lineages (Gray 29). Another finding as of late is the cases of plastid gene loss following divergence of red and green algae. By trying to map out these losses with phylogenetic trees, this can really help our ability to describe evolutionary relationships among compared plastids genomes. This will reveal the nature and timing of losses and then will showcase multiple independent losses of the same gene in different lineages. We will be able to discover the purpose to why plastids have loss certain genes when compared to more derived and newer genes. 7. Plants are a culmination of complex processes that often require specific substances. A lack of certain substances would hinder many functions of the plant and also be detrimental to the structure of the plant. Resin- A lack of this substance would put the plant at risk to invasion. At times when a plant is injured, they secrete resin to block up the injury. Since resin is normally under pressure, a rupture causes resin to flow out due to the reduced pressure and its viscosity. Without the flow of resin, the plant will be able to be infected by harmful fungi or insects. (Beck 189) Suberin- This substance frequently coats plant tissue near the vascular tissue and periderm. Without suberin there would be many instances of unregulated movement throughout the plant. Unregulated substances could be ions or water. In the periderm, the layers of phellem are coated with suberin to make it impermeable to water. If water were able to penetrate the periderm it would most likely lead to the erosion of dead mature cells that have the purpose of protecting the inside of the plant or supporting it. The weak structure of the plant could easily be fatal. Suberin is also characteristic of the Casparian strip in the endodermis. Without this Casparian strip the apoplastic movement of water and ions would not be regulated and could cause imbalances in water pressure and ion concentration. Besides the endodermis, the inner layers of many primary walls are suberized (called the suberin lamella). Transfer between cells in this instance would also be unregulated (Beck 284). Lignin- As lignin polymerizes with primary and secondary cells walls; it provides support to cells by reducing elasticity and increasing hardness and tensile compression. Lignin, like suberin, also makes the cell impermeable to water. Without these features, vertical growth of plants seems almost impossible. A lack of Lignin will make it impossible for the plant to counteract gravity and withstand movement from weather or animals. Lignin is vital to xylem and the uptake of water to cells is also important for growth. Xylem vessels would simply collapse without lignin, and tissues would not receive water. In general other tissues like phloem rely on the support of other lignified tissues or else they would collapse as well from stress. Hemicellulose- This matrix polysacride is important to cellulose microfibril deposition in cell walls. Fibrous hemicellulose xyloglucan coats the cellulose microfibrils and is then bonded to another hemicellulose via hydrogen bonding. Without it cellulose would not be deposited in the manner that gives primary cell walls their greatest potential of strength. As a result, the cell walls would be extremely weakened which could collapse the cell and even the plants overall structure. Hemicellulose also forms a cross link between pectin molecules (Beck 59). Pectin- Like hemicellose, pectin is vital to primary cell wall composition. In fact, the middle lamella is composed of only pectin. The pectic cell plate initiates the middle lamella during telophase, and the space between neighboring cells is created. Without the middle lamella, neighboring cells would not be stabilized together would be disastrous for cell to cell transfer of materials. The network of pectin integrates with the separate network of hemicellolose-cellulose. Starch- These grains are the immediate result of photosynthesis that develop within amyloplasts. Starch is largely for storage for the plant and is consumed for the plants own metabolic processes. Starch is also a large reason for herbivory and responsible for energy for herbivores as well. If a plant was not able to store energy, it would be have to be doing photosynthesis continuously and this is impossible during nighttime when light excitation is impossible. Plants would not be able to survive during times when rates of photosynthesis are not high, and plants encounter these situations quite frequently. Callose- The carbohydrate β-1,3-glucan is involved in a few places within a plant. Upon wounding callose encloses the sieve plates of sieve elements. It encases the plasmalemma and endoplasmic reticulum because these transverse pores. As a sieve element approaches the end of its functional life, callose accumulates and this is called Definitive Callose. Callose seems to be a barrier for plant cells so that current cell contents are not lost upon wounding and foreign substances are prevented from entering the cell and infiltrating the whole plant. Callose also develops in pollen tube as a way to protect the exine until sporopollenin completely coats the exine. The gametes of the plant might be degraded if not for the temporary protection of callose. Sporopollenin- This hydrophobic biopolymer coats the exine and makes it safe from degradation. The occurrence of degradation is frequent in cells and important substances have to be marked in order to be saved sometimes. With the protective sporopollenin, the exine would degrade and the reproduction of the plant would be extremely fragile. 8. Higher plants have a complex life that involves two phases: a diploid sporophyte phase and a haploid gametophyte stage. This revolving life is called alternation of generations. The large plant bodies that we usually see are sporophytes, which develop from fertilized zygotes. That zygote results from the fusion of two gametes, which are very small plant bodies. The development of fruit begins with meiosis occurring in both the anther and pistil. For anthers, the process is called microgametogenesis. It starts with microsporocytes that are contained in microsporangia, and microspores are created. These microspores are what develop into pollen grains, and upon germination they will turn into microgametophytes, the male part of fertilization. Each pollen grain has two cells, a generative cell and a tube nucleus. When germinated, the generative cell nucleus divides into two sperms. Both of these sperms have a nucleus and cytoplasm. Before fertilization, the two sperms are in contact with each other and the leading sperm is also in contact with the vegetative tube nucleus. It has more recently been found that the two sperms differ in their size and cellular contents in some taxa, and these differences can decide which sperm fuses with the egg cell. For megagametogenesis meiosis also occurs in megasporocytes, and one is contained in each of the developing ovules. Meiosis creates a linear tetrad of megaspores. Three of these spores degenerate and the remaining spore develop into the megagametophyte or embryo sac. Eight nuclei results from three mitotic divisions and as these divisions happen the original cell expands and elongates (Beck 360). Four of the nuclei migrate to each end of the developing embryo sac. At the micropylar end, one nucleus will become at least partially enclosed by a cell wall, and functions as an egg cell. The other two nuclei differentiate into synergids. These cells stay in contact with the egg cell and possess a filiform apparatus. The purpose of this apparatus is thought to be a transfer structure. At the opposite end of the embryo sac, the chalazal end, the three nuclei differentiate as antipodal cells. The remaining fourth nucleus at each end migrates to the center of the embryo sac. The central cell is then comprised of the two polar nuclei, the remaining cytoplasm, the synergids and the egg cell. The central cell along with the three antipodal cells makes up the mature female gametophyte. Not every instance of megagametogenesis processes this way, but this is the most frequent type in angiosperms called monosporic. After pollination and germination of the pollen grain on the stigma, the pollen tube proceeds through the style and into the locule of the carpel. It is impressive to see the distance for the pollen to grow in order to reach the embryo sac. It grows through the micropyle and as is does so, one of the synergids deteriorates to create an entrance for the pollen tube. When inside, the two sperms are released into the synergid. A recent study has shown that the surfaces of the sperms contain myosin and these motor complexes interact with microfibrils of F-actin in order to transport down the pollen tube (Beck 361). When inside the embryo sac, the leading sperm will fuse with the polar nuclei and forms the triploid endosperm nucleus. The following sperm fuses with the egg cell and forms a diploid zygote, and once this occurs double fertilization is complete. At this stage the ovule has an embryo sac, encased in a nucellus, and one or two integuments. This process occurs in gymnosperms with a few differences. One major difference is the reduction of structures in angiosperm reproduction as compared to gymnosperms. Ovules and microsporangia of gymnosperms are produced in large female and male cones respectively. In conifers, ovules are attached to the adaxial surface of ovuliferous scales, and the micropylar end of the ovule is facing the middle or cone axis. Microsporangia are borne on the abaxial surface of microsporophylls. The division of the developing megagametophyte occurs for much longer in gymnosperms (several months), and this creates a larger gametophyte. Following microsporogenesis (which is mostly similar to angiosperms), the conifer pollen grain is dispersed by wind and it lands in a pollination droplet, which will then retract and draw the pollen grain through the micropyle and contacts the megasporangium. Gymnosperms can also fertilize several eggs and several embryos will develop (called polyembryony). After this only one of the embryos will actually continue development and the others abort (Beck 354). Sources Beck, Charles. Plant Structure and Development. Cambridge University Press. Cambridge. 2005. Grey, Michael W. “The Evolutionary Origins of Plant Organelles”. Molecular Biology and Biotechnology of Plant Organelles. Springer. Dordrecht. 2004. No author. “Monocots versus Dicots: The Two Classes of Flowering Plants”. Internet. 5/05/06. http://www.ucmp.berkeley.edu/glossary/gloss8/monocotdicot.html