* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download L10 Ectopic-and-Molar-Pregnancy-Pregnancy
Breech birth wikipedia , lookup
Birth control wikipedia , lookup
Epidemiology of metabolic syndrome wikipedia , lookup
HIV and pregnancy wikipedia , lookup
Maternal health wikipedia , lookup
Women's medicine in antiquity wikipedia , lookup
Prenatal nutrition wikipedia , lookup
Prenatal testing wikipedia , lookup
Gestational diabetes wikipedia , lookup
Prenatal development wikipedia , lookup
Fetal origins hypothesis wikipedia , lookup
Maternal physiological changes in pregnancy wikipedia , lookup
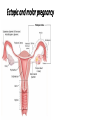
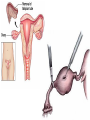
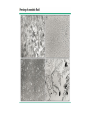
Pregnancy Complications Flip class-Monday • • • • • • • • Nausea and vomiting, Constipation, GERD during pregnancy Rh D Alloimmunization DM and GDM in pregnancy HTN, Pre-eclampsia and eclampsia in pregnancy VTE in pregnancy UTI in pregnancy Induction of Labor and Labor analgesia Postpartum Hemorrhage, Preterm Labor, Antenatal Glucocorticoid and PPROM Preterm Premature Rupture of Membranes • Ectopic and molar pregnancy DM during pregnancy & Gestational DM • GDM • Diagnosis of diabetes at 24 to 28 weeks of gestation • Overt DM • • • • Diagnosis at the first prenatal visit A1C ≥6.5 percent A fasting glucose ≥126 mg/dL A1C is 5.7 to 6.4 percent • Two hour 75 gram oral glucose tolerance test (GTT) Range of diagnostic criteria for gestational diabetes Approach Two step (100gram load) Two step (75gram load) One step (75gram load) Criteria* Fasting mg/dL One-hour mg/dL Two-hour mg/dL Three-hour mg/dL Carpenter and Coustan 95 (5.3 mmol/L) 180 (10.0 mmol/L) 155 (8.6 mmol/L) 140 (7.8 mmol/L) NDDG 105 (5.8 mmol/L) 190 (10.6 mmol/L) 165 (9.2 mmol/L) 145 (8.0 mmol/L) CDA 95 (5.3 mmol/L) 191 (10.6 mmol/L) 160 (8.9 mmol/L) WHO 92 to 125 (5.1 to 6.9 mmol/L) 180 (10.0 mmol/L) 153 to 199 (8.5 to 11 mmol/L) IADPSG 92 to 125 (5.1 mmol/L) 180 (10.0 mmol/L) 153 (8.5 mmol/L) • These thresholds are for diagnosis of gestational diabetes. Diagnosis of overt diabetes and diabetes in pregnancy are based on different criteria (eg, IADPSG: fasting blood glucose ≥126 mg/dL [7.0 mmol/L] is consistent with overt diabetes; WHO: two-hour glucose ≥200 mg/dL [11.1 mmol/L] following a 75-gram oral glucose load is consistent with diabetes in pregnancy). • NDDG: National Diabetes Data Group; • CDA: Canadian Diabetes Association; • WHO: World Health Organization; • IADPSG: International Association of Diabetes and Pregnancy Study Groups. Diagnostic criteria for the 100-gram three-hour GTT to diagnose gestational diabetes mellitus Plasma or serum glucose level Carpenter/Coustan Plasma level National Diabetes Data Group mg/dL mmol/L mg/dL mmol/L Fasting 95 5.3 105 5.8 One hour 180 10.0 190 10.6 Two hours 155 8.6 165 9.2 Three hours 140 7.8 145 8.0 100-gram oral glucose load is given in the morning to a patient who has fasted overnight for at least 8 hours. Glucose concentration greater than or equal to these values at TWO or more time points is a positive test. Two different classification schemes of GDM based upon results of the three-hour GTT results have been proposed. The Fourth International Workshop-Conference on Gestational Diabetes GTT values cited above are based upon the Carpenter and Coustan modification of earlier values. They are lower than those proposed by the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus and the National Diabetes Data Group (NDDG), which used cutoff values of 105, 190, 165, and 145 mg/dL (5.8, 10.6, 9.2, and 8.0 mmol/L), respectively. The values are lower because the thresholds derived from the older Somogyi-Nelson method of glucose analysis were corrected to account for the enzymatic assays currently in use. ADA criteria for the diagnosis of diabetes 1. A1C ≥6.5 percent. The test should be performed in a laboratory using a method that is NGSP certified and standardized to the DCCT assay.* OR 2. FPG ≥126 mg/dL (7.0 mmol/L). Fasting is defined as no caloric intake for at least eight hours.* OR 3. Two-hour plasma glucose ≥200 mg/dL (11.1 mmol/L) during an OGTT. The test should be performed as described by the World Health Organization, using a glucose load containing the equivalent of 75-gram anhydrous glucose dissolved in water.* OR 4. In a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥200 mg/dL (11.1 mmol/L). Risk factors • Pregnant women with any of the following characteristics appear • Personal history of impaired glucose tolerance or gestational diabetes in a previous pregnancy • Member of one of the following ethnic groups, which have a high prevalence of type 2 diabetes: Hispanic-American, African-American, Native American, South or East Asian, Pacific Islander • Family history of diabetes, especially in first degree relatives • Prepregnancy weight ≥110 percent of ideal body weight or BMI >30 kg/m2, significant weight gain in early adulthood and between pregnancies, or excessive gestational weight gain • Maternal age >25 years of age • Previous delivery of a baby >9 pounds (4.1 kg) • Previous unexplained perinatal loss or birth of a malformed infant • Maternal birthweight >9 pounds (4.1 kg) or <6 pounds (2.7 kg) • Glycosuria at the first prenatal visit • Medical condition/setting associated with development of diabetes, such as metabolic syndrome, polycystic ovary syndrome (PCOS), current use of glucocorticoids, hypertension Timing of screening/testing • 1st first prenatal • In the absence of early screening/testing or if early screening/testing is negative, universal screening is performed at 24 to 28 weeks of gestation Management • Medical nutritional therapy, • Self-monitoring of blood glucose levels • Awakening and one or two hours after each meal • Insulin therapy, when needed rather than oral anti-hyperglycemic agents during pregnancy • Glyburide or metformin is a reasonable alternative for women who refuse to take, or are unable to comply with, insulin therapy. • metformin results in a lower rate of macrosomia than use of glyburide, metformin users are more likely to require supplemental insulin to maintain euglycemia than glyburide users. The long-term effects of transplacental passage of oral anti-hyperglycemic agents are not known. • Target: 95/130-140/120 • Initiate: 95/13-140/120 • fasting BG ≥95 mg/dL/one-hour postprandial BG ≥130 to 140 mg/dL/two-hour BG >120 mg/dL • Women with GDM be tested postpartum and that they receive screening at least every three years thereafter HTN in pregnancy • Preeclampsia-eclampsia • Syndrome of new onset of hypertension and either proteinuria or end-organ dysfunction most often after 20 weeks of gestation in a previously normotensive woman • Eclampsia is diagnosed when seizures have occurred. • Chronic (preexisting) hypertension • • • • ≥140 mmHg and/ ≥90 mmHg Antedates pregnancy, Present before the 20th week of pregnancy, Persists longer than 12 weeks postpartum. Criteria for the diagnosis of preeclampsia Systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg on two occasions at least four hours apart after 20 weeks of gestation in a previously normotensive patient If systolic blood pressure is ≥160 mmHg or diastolic blood pressure is ≥110 mmHg, confirmation within minutes is sufficient and Proteinuria ≥0.3 grams in a 24-hour urine specimen or protein (mg/dL)/creatinine (mg/dL) ratio ≥0.3 Dipstick ≥1+ if a quantitative measurement is unavailable In patients with new-onset hypertension without proteinuria, the new onset of any of the following is diagnostic of preeclampsia: Platelet count <100,000/microliter Serum creatinine >1.1 mg/dL or doubling of serum creatinine in the absence of other renal disease Liver transaminases at least twice the normal concentrations Pulmonary edema Cerebral or visual symptoms HTN in pregnancy • Preeclampsia-eclampsia superimposed upon chronic hypertension • Gestational hypertension • elevated blood pressure first detected after 20 weeks of gestation in the absence of proteinuria or other diagnostic features of preeclampsia. • May progress to pre-eclampsia • May progress to chronic HTN HTN in pregnancy • Treatment of severe hypertension: ok • mild to moderate hypertension: not ok • Angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, and direct renin inhibitors are contraindicated at all stages of pregnancy Chronic (preexisting) hypertension • uncomplicated mild primary hypertension: • Not initiating antihypertensive • Initiate or resume antihypertensive • ≥150 mmHg/ 95 to 99 mmHg • lower pressures and signs of hypertensive end-organ damage • Antihypertensive therapy • methyldopa or labetalol • A long-acting calcium channel blocker (eg, nifedipine) can be added if needed. • Target: 140 to 150 mmHg/ 90 to 100 mmHg. Preeclampsia • avoiding antihypertensive therapy for mild hypertension associated with preeclampsia • Treatment of severe hypertension to prevent maternal cerebrovascular complications. • Initiate antihypertensive at ≥150/ ≥100 mmHg. • Initiate treatment at a lower threshold in younger women whose baseline blood pressure was low, and in those with symptoms that may be attributable to elevated blood pressure (eg, headache, visual disturbances, chest discomfort). • American College of Obstetricians and Gynecologists (ACOG) • Treatment at ≥160 mm Hg. • Acute blood pressure therapy • labetalol IV or hydralazine IV. • Target blood pressure goal is systolic pressure of 140 to 150 mmHg and diastolic pressure of 90 to 100 mmHg. Options for breastfeeding mothers • Beta-blockers and alpha/beta-blockers • Propranolol, metoprolol, and labetalol • have the lowest transfer into milk • Calcium channel blockers • Diltiazem, nifedipine, nicardipine, and verapamil • The American Academy of Pediatrics (AAP) compatible • Angiotensin converting enzyme (ACE) inhibitors • Captopril and enalapril • AAP compatible • Diuretics • AAP compatible Eclampsia • Occurrence of new-onset, generalized, tonic-clonic seizures or coma in a woman with preeclampsia. • Preeclampsia/eclampsia remains a common cause of maternal morbidity and death • Clinical presentation and findings • • • • hours before the initial seizure Hypertension (75 percent) Headache (persistent frontal or occipital headaches or thunderclap headaches) (66 percent) Visual disturbances (scotomata, loss of vision [cortical blindness], blurred vision, diplopia, visual field defects [eg, homonymous hemianopsia], photophobia) (27 percent) • Right upper quadrant or epigastric pain (25 percent) • Asymptomatic (25 percent) • Ankle clonus is also a common finding Eclampsia • Management • If the seizure is witnessed, maintaining airway patency and preventing aspiration are the initial priority • Prevention of maternal hypoxia and trauma • Treatment of severe hypertension, if present • Prevention of recurrent seizures • Evaluation for prompt delivery Eclampsia • Management • Treatment of severe hypertension, if present • to prevent stroke, which accounts for 15 to 20 percent of deaths from eclampsia. • Initiating: ≥160 mmHg /105 to 110 mmHg???? • Drug choice and dose, and target blood pressure are the same as in preeclampsia Eclampsia • Management • Prevention of recurrent seizures • The anticonvulsive drug of choice is magnesium sulfate. • Safer and more effective than phenytoin, diazepam, or lytic cocktail (ie,chlorpromazine, promethazine and pethidine) for prevention of recurrent seizures in eclampsia • Loading dose • 6 g intravenously over 15 to 20 minutes • Maintenance dose • 2 g / hr • Management of persistent seizures • Diazepam and lorazepam Eclampsia • Management • Delivery Only curative treatment • Cesarean delivery is a reasonable option for women less than 32 to 34 weeks of gestation with an unfavorable cervix. Prevention of Rh(D) alloimmunization in pregnancy • Rh(D)-negative pregnant women • Antibody screen at the 1st prenatal visit. • Negative • repeat screen at 28 weeks of gestation is optional • Noninvasive fetal RHD genotyping is the standard approach in some countries Prevention of Rh(D) alloimmunization in pregnancy • Rh(D)-negative Pregnants whose fetus is/may Rh(D)-positive: • Administration of anti-D immunoglobulin early in the third trimester • 300 micrograms at 28 weeks of gestation • 100 to 120 micrograms at 28 and 34 weeks Prevention of Rh(D) alloimmunization in pregnancy • Antenatal anti-D immunoglobulin • Increased risk of fetomaternal hemorrhage • • • • • • • • • • • miscarriage, abortion, ectopic pregnancy, multifetal reduction, amniocentesis, chorionic villus sampling, blunt abdominal trauma, external cephalic version, antepartum bleeding, and fetal death. 300 micrograms as soon as possible within 72 hours of the event Prevention of Rh(D) alloimmunization in pregnancy • Ongoing risk for fetomaternal hemorrhage • Repeat dosing • chronic placental abruption or placenta previa with intermittent vaginal bleeding • Serial determinations of the maternal indirect Coombs every three weeks with repeat dosing if it is found to be negative. Prevention of Rh(D) alloimmunization in pregnancy • Anti-D immunoglobulin within 72 hours of delivery of an Rh(D)positive infant • 300 micrograms • Additional doses • Excessive fetomaternal hemorrhage • If inadvertently omitted after delivery • as soon as possible • Partial protection is afforded with administration within 13 days of the birth • May be an effect as late as 28 days after delivery Prevention of Rh(D) alloimmunization in pregnancy • Management of pregnancies complicated by alloimmunization • intrauterine fetal transfusions • Investigational • maternal plasmapheresis • intravenous immune globulin therapy Ectopic and molar pregnancy • Definition • Extrauterine pregnancy. • Fallopian tube (98 percent) • Symptoms • Abdominal pain • Vaginal bleeding • > 50 percent of women are asymptomatic • Evaluation • transvaginal ultrasound (TVUS) examination • Quantitative human chorionic gonadotropin (hCG) level • measured serially every 48 to 72 hours • hCG discriminatory zone • Mostly 1500 to 2000 IU/L • hCG is below the discriminatory zone • Serial hCG is rising abnormally (does not increase by at least 53 percent in 48 hours OR doubling in 72 hr • Diagnosis is made based upon the hCG pattern. • hCG is above the discriminatory zone • Diagnosis by ultrasound Diagnosis Algorithm • The fetal pole • Thickening on the margin of the yolk sac of a fetus during pregnancy. • Usually identified • 8 weeks with abdominal US • 6 weeks with vaginal ultrasound imaging • Quite normal for the fetal pole to not be visible until about 9 weeks • Between 5 ½ to 6 ½ weeks, a fetal pole or even a fetal heartbeat may be detected by vaginal ultrasound. • yolk sac attaches outside the developing embryo • Connected to the umbilical cord by a yolk stalk. • Acts as the preliminary circulatory system and is eventually absorbed into the gut of the embryo. adnexal mass • An adnexal mass is a lump in tissue of the adnexa of uterus • structures closely related structurally and functionally to the uterus such as the ovaries, fallopian tubes, or any of the surrounding connective tissue. • Adnexal masses can be benign or cancerous, and they can be categorized as simple or complex The higher the hCG, The higher the rate of failure • MTX treatment optimal • • • • • Hemodynamically stable Have no renal, hepatic, or hematologic disorders Able and willing to comply with post-treatment monitoring Pretreatment serum hCG concentration less than 5000 mIU/mL Tubal size of less than 3 to 4 cm and no fetal cardiac activity (these are not independent predictors of MTX treatment success) • Tubal pregnancy If treated with MTX • single-dose over multiple-dose regimen • Interstitial pregnancy • Multi-dose regimen • Surgery • Urgent surgical treatment • Women preference Treatment overview • Ruptured ectopic pregnancy or if patient is hemodynamically unstable • Surgery is necessary • Patients with ectopic pregnancy who are clinically stable • both surgery and methotrexate therapy may be appropriate first-line therapy if unruptured ectopic pregnancy http://www.dynamed.com/topics/dmp~AN~T115772/Ectopic-pregnancy#Treatment Treatment overview • Surgery • laparoscopy is often the preferred method • options include • salpingectomy or salpingostomy • At least as effective as intramuscular methotrexate in women with unruptured ectopic pregnancies (level 2 [mid-level] evidence) • single dose of methotrexate following salpingostomy may prevent persistent ectopic pregnancy in women with unruptured ectopic pregnancy (level 2 [mid-level] evidence) http://www.dynamed.com/topics/dmp~AN~T115772/Ectopic-pregnancy#Treatment Treatment overview • Medical treatment with methotrexate • No studies found comparing intramuscular methotrexate to placebo • 3 different dosing protocols • single-dose regimen of intramuscular methotrexate may not be as effective in eliminating ectopic pregnancy as laparoscopic salpingostomy (level 2 [mid-level] evidence) • 2-dose regimen reported to be successful in 87% of cases (level 3 [lacking direct] evidence) • multiple-dose methotrexate not clearly more effective than single-dose methotrexate for ectopic pregnancy (level 2 [mid-level] evidence) • multi-dose intramuscular methotrexate not clearly more effective than surgical management in hemodynamically stable women with ectopic pregnancy (level 2 [midlevel] evidence) http://www.dynamed.com/topics/dmp~AN~T115772/Ectopic-pregnancy#Treatment Treatment overview • expectant management • Patient is asymptomatic, • evidence of spontaneous resolution is occurring, and • patient accepts the risk of rupture • Spontaneous resolution reported to occur in 48%-88% of ectopic pregnancies http://www.dynamed.com/topics/dmp~AN~T115772/Ectopic-pregnancy#Treatment Homework • What is the prevalence of ectopic pregnancy among Jordanians? • PMID: 21077507 • 1/180-1/150 Molar pregnancy • Gestational trophoblastic disease GTD • Benign • Hydatidiform mole • Complete mole • Karyotype diploid • Partial mole • Karyotype triploid • Malignant • • • • Invasive mole, Choriocarcinoma, Placental site trophoblastic tumor, and Epithelioid trophoblastic tumor. Molar pregnancy • Risk factors • Extremes of maternal age • ≤15 and >35 years • History of previous mole • S&S • • • • Elevated serum hCG Vaginal bleeding, Pelvic discomfort. Enlarged uterus Molar pregnancy • Diagnosis • Serum hCG • hCG is high as >100,000 mIU/mL • Pelvic ultrasound Karyotype triploid Karyotype Diploid Treatment • Uterine evacuation + suction curettage rather than medication-only • Medications • (misoprostol, mifepristone, oxytocin) • age ≥40 years and have completed childbearing • Hysterectomy rather than uterine evacuation • Patients at high risk for GTN (neoplasia) • Hysterectomy • Prophylactic chemotherapy • MTX or actinomycin D • Follow-up of treatment • Serial weekly serum hCG • Reliable contraception during the entire interval of hCG monitoring. • Rh(D) immune globulin • Patients who are Rh(D)-negative should receive anti-Dimmune globulin at the time of treatment because the Rh(D) factor is expressed on trophoblast. Treatment • Suction dilatation and curettage is the preferred treatment • (Strong recommendation). • Monitor for malignant postmolar gestational trophoblastic disease after surgical management of molar pregnancy • (Strong recommendation). • Serial monitoring of human chorionic gonadotropin (hCG) to screen for postmolar gestational trophoblastic disease is preferred over routine prophylactic chemotherapy after evacuation of molar pregnancy in patients for whom follow-up is likely, although prophylactic chemotherapy may decrease risk of postmolar gestational trophoblastic disease in high-risk patient, especially if poor follow-up is likely • (Weak recommendation). • After diagnosis of molar pregnancy, advise patient to use contraception and avoid pregnancy until hCG levels remain low (documented remission) for 6-12 months • (Strong recommendation). Preterm Labor • Early signs and symptoms • • • • • • Non-specific Menstrual-like cramping; mild, irregular contractions; low back ache; pressure sensation in the vagina; vaginal discharge of mucus, which may be clear, pink, or slightly bloody (ie, mucus plug, bloody show) Preterm births • Early (<34 week) • Late (34 to 36 weeks) • Spontaneous • 70-80% • Preterm labor 40-50% • PPROM 20-30% • Initiated by a clinician Preterm Labor • Diagnosis • Regular painful uterine contractions accompanied by cervical dilation and/or effacement (cervix stretches and gets thinner). • 34-35 weeks of gestation is the threshold at which perinatal morbidity and mortality are too low to justify the potential maternal and fetal complications and costs associated with inhibition of preterm labor, which only results in short-term delay in delivery Preterm Labor • Discharge home • ≥34 weeks of gestation, • Without progressive cervical dilation and effacement after an observation period of four to six hours • Fetal well-being is confirmed • <34 weeks of gestation+cervical length <3 cm • transvaginal ultrasound • analysis of cervicovaginal fetal fibronectin Management • Antenatal corticosteroid therapy for reduction of neonatal morbidity and mortality from preterm delivery • Tocolytic drugs for up to 48 hours to delay delivery so that betamethasone given to the mother can achieve its maximum fetal effect. • Magnesium sulfate for pregnancies at 24 to 32 weeks of gestation. • In utero exposure to magnesium sulfate provides neuroprotection against cerebral palsy and other types of severe motor dysfunction in offspring born preterm. • Antibiotic therapy has no role in the treatment of acute preterm labor in the absence of a documented infection or GBS prophylaxis Inhibition of acute preterm labor • Tocolytics to delay in delivery for 48 hours • So that antenatal corticosteroids can be administered and achieve their maximal effect; • Allow safe transport of the mother, if indicated, to a facility that can provide an appropriate level of neonatal care if the patient delivers preterm; and • prolong pregnancy when there are underlying, self-limited causes of labor, such as abdominal surgery, that are unlikely to cause recurrent preterm labor. Inhibition of acute preterm labor • 24 to 32 weeks of gestation • Indomethacin as first-line therapy for labor inhibition • C/I • • • • • • maternal platelet dysfunction or bleeding disorder, hepatic dysfunction, gastrointestinal ulcerative disease, renal dysfunction, or asthma Gestations over 32 weeks and caution against their use for more than 72 hours because of concern about premature narrowing or closure of the ductus arteriosus • Nifedipine • 24 to 32 weeks + C/I to Indomethacin • Terbutaline Inhibition of acute preterm labor • 32 to 34 weeks of gestation • Nifedipine as the first-line therapy • terbutaline • avoid concurrent use of tocolytic drugs Inhibition of acute preterm labor • Less effective tocolytic drugs • Oxytocin receptor antagonists • Atosiban • Magnesium sulfate • Nitric oxide donors • Ineffective • • • • • bedrest, hydration, sedatives, antibiotics, and progesterone supplementation Threatened abortion Postpartum Hemorrhage • Definition • ≥500 mL after vaginal birth or • ≥1000 mL after cesarean delivery. • Primary PPH occurs in the first 24 hours after delivery • Secondary PPH occurs 24 hours to 12 weeks after delivery • Causes • atony, • trauma, and • acquired or congenital coagulation defects Postpartum Hemorrhage • Management • Depending on the cause and whether the patient has had a vaginal birth or cesarean delivery. • Traumatic, hemorrhaging lesions are managed surgically • Coagulopathy is managed medically, with replacement of blood products. • Atony depends on the route of delivery PPROM • Membrane rupture before the onset of uterine contractions • Preterm Premature Rupture of Membranes • Prelabor rupture of membranes • before 370/7thsweeks of gestation. • 3% • 30% of preterm births PPROM • Risk factors • Similar to those for preterm labor • Strong association • • • • PPROM in a previous pregnancy, genital tract infection, antepartum bleeding, and cigarette smoking Risk factors for preterm birth Risk factors for preterm birth PPROM • The diagnosis of PPROM is clinical, • Visualization of amniotic fluid in the vagina of a woman who presents with a history of leaking fluid. • Laboratory tests (eg, Nitrazine and fern or Amnisure) are used for confirmation in cases of clinical uncertainty. Pregnancy complications associated with preterm premature rupture of membranes (PPROM) • (A) Typical ferning pattern of dried amniotic fluid (400). (B, C) Urine and amniotic fluid can be distinguished by microscopic examination of a droplet of the fluid spread and dried on a microscope slide. The proteins in amniotic fluid give the appearance of ferning (B) that is not observed with urine (C). (D) Ferning pattern from amniotic fluid. CDC algorithm for screening for GBS in PROM before 37 weeks of gestation PPROM management • Expeditious delivery • • • • intrauterine infection, abruptio placentae, nonreassuring fetal testing, or a high risk of cord prolapse is present or suspected PPROM management • Stable patients with PPROM <34 weeks • Expectant management • + A course of antenatal corticosteroids to enhance fetal lung maturation in pregnancies less than 34 weeks of gestation • +Prophylactic antibiotics • ampicillin 2 g intravenously every 6 hours for 48 hours, followed by amoxicillin (500 mg orally three times daily or 875 mg orally twice daily) for an additional five days. • one dose of azithromycin (one gram orally) at the time of admission and repeat the dose five days later. • Confirmed gestational age • Delivery at ≥34 weeks of gestation without assessment of pulmonary maturity • Gestational age is uncertain • Confirm lung maturity before delivery • delivery at 36 weeks, assuming the mother and fetus are stable Antenatal Glucocorticoid • Improvement in neonatal lung function by enhancing maturation • Reduces the incidence of • • • • • respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, sepsis, and neonatal mortality by approximately 50 percent. Antenatal Glucocorticoid • Administration of antenatal corticosteroids to pregnant woman • 23 to 34 weeks of gestation • Increased risk of preterm delivery within the next seven days • Rupture membranes or • Receiving tocolysis for active preterm labor, • Delivery for maternal or fetal indications is anticipated within the next seven days. Antenatal Glucocorticoid • 230/7ths weeks of gestation the lower limit • Earlier administration in the 22nd week is reasonable if aggressive neonatal intervention is planned Antenatal Glucocorticoid • A course of antenatal corticosteroids consists • betamethasone 12 mg IM every 24 hours for two doses • Dexamethasone 4 doses of 6 mg IM 12 hours apart. Antenatal Glucocorticoid • Women delivered at 340/7ths to 386/7ths • Theoretical long-term hazards of steroid administration, particularly adverse neurodevelopment • Cesarean delivery at 340/7ths to 366/7ths weeks • May give first course of antenatal corticosteroids • 340/7ths to 366/7ths weeks who have already received a course of antenatal corticosteroids, or who are expected to deliver vaginally, or whose likelihood of delivery within the next seven days is uncertain, we suggest not administering a course of antenatal corticosteroids • Repeat courses of steroids have not be studied after 34 weeks, transient tachypnea of the newborn is less common after labor and vaginal birth, and most women with threatened preterm labor do not deliver preterm. • Inadequate data • • • • number of courses that are safe for the fetus, the appropriate time interval between courses, the optimal dose for repeated courses of therapy, or the full ramifications of the single course approach to therapy • Single course of salvage (rescue, booster) • Risk of delivery within the next seven days, • More than two weeks have elapsed since the initial course of antenatal corticosteroid therapy, • Gestational age at administration of the initial course was <28 weeks of gestation • Two dose course is also reasonable. Induction of Labor • Techniques for stimulating uterine contractions to accomplish delivery prior to the onset of spontaneous labor. • Indicated • Continuing the pregnancy is thought to be associated with greater maternal or fetal risk than intervention to deliver the pregnancy • Avoid elective induction of labor at term • Modified Bishop scoring system • ≥8 suggests the chances of having a vaginal delivery are good and the cervix is considered favorable or ripe for induction. • ≤6 the chances of having a vaginal delivery are low and the cervix is considered unfavorable or unripe for induction. Induction of Labor • Women with favorable cervices • Oxytocin with early rather than amniotomy alone • Artificial rupture of membranes (AROM) • low-dose oxytocin protocol • Oxytocin alone without amniotomy is also reasonable • High-dose oxytocin regimens • Decrease the time from admission to vaginal delivery, • Does not appear to decrease the incidence of cesarean delivery • Higher rate of tachysystole than low-dose regimes Oxytocin infusion protocols Regimen Starting dose, milliunits/ minute Low-dose Alternative low-dose 0.5 to 1 1 to 2 High-dose 6 Alternative high-dose 4 Dosage interval, minutes Incremental increase, milliunits/minute 1 2 6 The incremental increase should be reduced to 3 milliunits/minute if hyperstimulation is present, and reduced to 1 milliunit/minute if recurrent hyperstimulation. Some clinicians limit to a maximum cumulative dose of 10 units and a maximum duration of six hours. 4 30 to 40 15 to 30 15 to 40 15 Side effects of oxytocin • Maternal • cardiovascular instability • • • • hypotension, tachycardia, myocardial ischemia, arrhythmias • Nausea, vomiting, headache, and flushing • Rarely, large doses have caused water retention, leading to hyponatremia. • Neonatal • Hyperbilirubinemia? Ripening the unfavorable cervix prior to induction • Two major techniques • Mechanical (physical) interventions • Insertion of catheters or cervical dilators • Application of cervical ripening agents • Prostaglandins • Misoprostol (Cytotec®) • PGE1 analog • 100 mcg and 200 mcg tablets, breakable • Oral and vaginal routes • Dinoprostone • PG E2 • for vaginal insertion, and tablet for oral administration Labor analgesia Labor analgesia • Low concentrations of the local anesthetics bupivacaine and ropivacaine produce greater sensory than motor block • Fentanyl and sufentanil are used interchangeably to provide labor analgesia • Provide only about 90 minutes of pain relief. • Do not provide reliable analgesia for the late first stage or second stage of labor. • Combination of fentanyl or sufentanil and a low concentration of bupivacaine or ropivacaine. Labor analgesia • Epinephrine • Potentiate analgesia • Decrease local anesthetic requirements, • Enhances motor block, • Cesarean delivery under neuraxial anesthesia • Local anesthetic + epinephrine