* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Homework05 n large samples
Survey
Document related concepts
Transcript
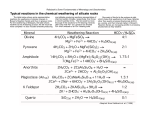
Homework Questions for Lecture 5 ES 1000 Use these questions to test your knowledge of Lecture 5 and related reading. A. Short answer: 1. ___________ is the breaking down of rocks into rock fragments. 2.____________ is the transporting of rock fragments. 3. __________ weathering can change the size and shape of rock structures. 4. _______ wedging breaks up large rocks because water increases in volume when it freezes. 5. The primary cause of mechanical _____________ is the removal by erosion of overlying rock and soil. 6. The single most important factor controlling the rate of chemical weathering is ___________. 7. The most effective type of mechanical weathering in a summertime coastal environment would be ______ crystal growth. 8. Different kinds of _______ within a rock expand at different rates when heated. 9. The outside of a large rock experiences more temperature __________ than the inside of the rock. 10. The addition of water to the surface of a rock influences the rate of __________________. B. Match the terms 1. Abrasion _____ a. deeply weathered, shallow nutrients 2. Frost wedging ______ b. red iron oxide topsoil and subsoil 3. Carbonic Acid____ c. weathers basalt, leaving rust behind 4. Mechanical Exfoliation ____ d. dissolution of limestone 5. Hydrolysis ____ e. exposed rock expands upward 6. Rainforest soil _____ f. turns feldspar into clay 7. Oxidation _____ g. significant in deserts 8. Laterite _____ h. beach weathering 9. Thermal expansion & contr. ____ i. ice in cracks breaks rocks 10. Salt crystal growth._____ j. breaks rocks in streams, glaciers 11. Pedocal soil _____ k. soil with layer of calcite "caliche" C. True or False? 1. The B horizon generally lies above the E horizon. True or False? 2. The single most important factor controlling the rate of chemical weathering is water. True or False? 3. In chemical weathering, water carries ions to reaction sites, participates in the reactions, and carries away products of the reactions. True or False? 4. The dissolution of limestone to form caves results from water combining with carbon dioxide to form carbonic acid. True or False? 5. In geology, the term “oxidation” often refers to the chemical combination of a mineral’s ions with oxygen. True or False? 6. Hydrolysis refers to the displacement of a mineral’s ions by the H+ or OH- ions from water. True or False? 7. In general, chemical weathering would occur most rapidly in a cool, dry climate. True or False? 8. Carbon dioxide is added to the atmosphere by volcanic eruptions. True or False? 9. Soil is the upper portion of regolith, containing both minerals and organic matter, that supports the growth of plants. True or False? 10. The color of the E horizon results from humus that has leached downward from the A horizon. True or False? D. Multiple choice: 1. Plants promote chemical weathering by: (a) producing organic acids [upon decay]. (Alternate version, [during life]) (b) consuming carbon dioxide in photosynthesis. (c) shading the ground surface from rainfall. (d) shading the ground surface from intense sunlight. 2. Animals influence the rate at which chemical weathering takes place primarily by: (a) secreting acid that dissolves rocks and minerals. (b) increasing the exposure of rocks and minerals to weathering agents. (c) grinding rocks and minerals into smaller pieces. (d) ingesting the minerals that are necessary for nutrition. 3. Stability (resistance to weathering) of silicate minerals follows the reverse of their order of crystallization (Bowen’s Reaction Series). The correct sequence of stability for the minerals below would be (most stablefirst, least stable-last): (a) olivine, pyroxene, mica, amphibole, quartz. (b) pyroxene, olivine, amphibole, mica, quartz. (c) mica, pyroxene, amphibole, olivine, quartz. (d) quartz, mica, amphibole, pyroxene, olivine. Comment: Low temperature, 3-D framework quartz is most resistant because it has strong covalent bonds everywhere. 4. Clay minerals are formed predominately from the hydrolysis of: (a) feldspars. (b) nonsilicate minerals. (c) quartz. (d) metal ores. 5. Nutrient-poor soils are derived from a parent material that resists chemical weathering, such as: (a) limestone. (b) sandstone. (c) granite. (d) basalt. (Hint: Look at question 3. Which of these rocks is made of the most resistant mineral?) 6. In which of the following locations would a rich soil accumulate most quickly? (a) A relatively flat topography in a moist, mid-latitude climate. (b) A steep topography in a dry, cold climate. (c) A relatively flat topography in a very dry, hot climate. (d) A steep topography in a moist, mid-latitude climate. 7. In a temperate region, the most distinctive feature of the O horizon of a soil is: (a) the abundance of finely ground rock particles. (b) the abundance of both organic matter and living organisms. (c) its inability to maintain fertility because of constant leaching of materials to lower soil horizons. 8. The most distinctive feature of the E horizon is: (a) its dark color from the accumulation of humus. (b) its mixture of both large and small rock particles. (c) its lack of both organic matter and soluble minerals. (d) its high concentration of oxides E. Short answers. a. How does oxidation work? b. What type of rock is vulnerable? (Hint: what type of rock is made of the minerals that are least stable here at the surface?) c. How does hydrolysis work? d. What minerals are vulnerable to hydrolysis? e. How do plants use hydrolysis? f. What minerals result from the hydrolysis of Feldspars? g. Why are clays easy for streams to move down to the sea? h. How does dissolution work? i. What happens to Calcite (CaCO3) when you drop very dilute acid on it? j. How is mildly acid rain formed without pollution? k. What type of rock is vulnerable to dissolution? ______________________________________________________ Instructor's Comment: These three processes, oxidation, hydrolysis, and dissolution, break the minerals in rocks down to quartz grains, clay, and a lot of ions dissolved in water. Initially these three processes may form soils, which are vulnerable to erosion. Ultimately the dissolved ions in water and the solid quartz and clay grains can be transported to the sea. The dissolved ions make the ocean "salty". In the seabed, the dissolved ions in water can combine (bond) with other dissolved atoms and form new solids that cement sediment particles together. Important cements are iron oxides Fe2O3, silica SiO2, and calcite CaCO3. These hold together the grains of quartz, clay and other minerals to form sedimentary rocks. F. Standard Error of the Difference between Means Last time you calculated the sample standard deviation, S. Let’s put it to work. At Rutgers Research Farm 3, they plant varieties of Potato, Solanum tuberosum, and do not use pesticides, relying instead on predatory insects and plant toxins to control pests. There are Colorado Potato Beetles, Leptinotarsa decemlineata, on their plants. The larvae eat potato plant leaves, slowing the growth of the edible potato tubers people use for food. We’ll compare two varieties of Potato. Is there a difference between the average weights of Colorado Potato Beetle larvae feeding on Potato variety 'Katahdin', versus those on Potato 'Dark Red Norland' ? Heavy larvae suggest that variety has leaves that are edible, not poisonous. Fourth instar Colorado Potato Beetle larvae were weighed from Katahdin plants (N= 31, mean weight X1 = 0.189 grams, standard deviation S1 = 0.030), and from Dark Red Norland (N = 38, X2 = 0.134, S2 = 0.035). Are the means really different, or do they simply appear different due the small samples? We may use the "Standard Error of the Difference between Means" test. S.E. X1 - X2 = s12 + s22 N1 N2 RULE: This is a z-score. If the difference between the two means is more than 1.96 times the standard error of the difference, S.E. X1 - X2, the difference between the two means is significant (the probability P of this event is less than or equal to .05) a. Calculate the difference between the means X1 - X2. _________ grams b. Calculate S.E. X1 - X2 ____________ c. Compare the size of difference between the means and S.E. X1 - X2. Use the Rule above. Is the standard error of the difference between the two means statistically significant? Choose one: (there is a statistically significant difference in the mean size of larvae / there is no statistically significant difference in the mean size of larvae)