* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Integrated Clinical Information Systems for Traditional and Non-traditional Users
Survey
Document related concepts
Transcript
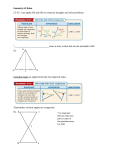
ROSENBERG I Clinical Info Systems INTEGRATED CLINICAL INFORMATION SYSTEMS FOR TRADITIONAL AND NON-TRADITIONAL USERS Martin J. Rosenberg, PhD, MAJARO lNFOSYSTEMS, INC. been a part of the drug development process. We call a computer system for the collection, retrieval, and analysis of clinical trial information, a Clinical Information System or CIS. The current concept of a Clinical Information System is shown in Figure 1. Physicians who conduct clinical trials of new drugs transmit the raw data to the sponsor on case report forms (CRFs) which are then entered into a database management system (DBMS). The data from the DBMS is transferred to a data analysis package for statistical analysis and computer generation of tables and graphs which are then incorporated into reports which become part of the NDA Although no standard DBMS has emerged in the U.S. pharmaceutical industry, SAS has long been the standard software used for statistical analysis, report generation, and production of summary tables and graphs. ABSTRACT Increasingly, pharmaceutical companies are attempting to enhance productivity by expanding the scope of their clinical information systems to embrace nontraditional users such as: investigators, in-house clinical staff, and FDA reviewers. As these systems become commonplace, companies are confronted with the prospect of having up to five different computer systems, each with incompatible data structures: a remote data entry system; a database management system for traditional data entry; a clinical data review system for use by in-house clinical staff; a statistical analysis system; and a computer assisted NDA review system (CANDA). This paper describes the emerging features of SAS software, including Version 6 enhancements such as screen control language, host windowing environments, multi-vendor architecture, multiple engine architecture, indexing, PROC ACCESS, and PROC SQL; and explores their role as a connectivity tool in constructing remote data entry, CANDA, and clinical data review systems in order to develop an integrated approach to clinical information systems. DBMS I STATISTICAL ANALYSIS I CLINICAL INFORMATION SYSTEMS AUTOMATED TABLE AND GRAPH GENERATION Before a new drug can be introduced into the marketplace, pharmaceutical firms must undertake a research process which can frequently take five or more years. The company collects information about the safety of the drug in both animals and humans; its efficacy in the diseases to be treated; the stability of the drug (how long it can remain on the shelf without degrading); the pharmacokinetics of the drug, i.e., how it is metabolized in humans; and the ability of the company to manufacture the drug in production quantities. Figure 1: Current CIS In an effort to better manage the volume of information and to reduce the length of time required to introduce a drug in the United States, the Food and Drug Administration, in conjunction with the pharmaceutical industry, has been experimenting with ways of using computer technology to facilitate the NDA review process. Such a computer system is called a As can be imagined from the magnitude of information that must be collected, computer systems have long 21 ROSENBERG I Clinical Info Systems Computer Assisted NDA Review system or CANDA. Participants in the CANDA experiments have reported that similar systems would be of use not only by FDA reviewers, but by the medical staff of pharmaceutical corporations. We designate systems for use by medical staff to monitor ongoing clinical trials, Clinical Data Review Systems. Simultaneously, there has been much interest in computer systems that permit the investigator to enter data and the sponsor to remotely monitor the trial. The intent of such systems is to collect more timely and accurate information. We call such systems Remote Data Entry and Monitoring systems or RED EM. computer assisted NDA review system (CANDA). The introduction of separate, incompatible systems could adversely impact productivity. Companies must maintain duplicate databases for each structure (with the attendant risk of inconsistencies between the databases) and must either train the staff in the operation of multiple systems or maintain separate staffs for each technology. Consequently, it would be desirable to integrate these five functions. Since SAS software is already in common use throughout the pharmaceutical industry, this paper explores the emerging capabilities of SAS and the role they can play in producing the integrated CIS of tomorrow. The remainder of this paper explores the use of the current Version 5.18 release of SAS on mainframes and the emerging features of Versions 6.03 and 6.06. With the introduction of these three new classes of users (investigators, in-house medical staff, and FDA reviewers) future clinical information systems will resemble Figure 2. Not every study will require all the facilities of the future CIS's. In particular, REDEM is likely to be used in selected studies only. However, pharmaceutical firms will increasingly expect to have these capabilities at their disposal. As such systems become commonplace, companies will be confronted with the prospect of having up to five different computer systems, each with incompatible data structures: a remote data entry and monitoring system; a database management system for traditional data entry; a clinical data review system for use by in-house clinical staff; a statistical analysis system; and a CLINACCESS™ CLINACCESS Version 5 is a Clinical Data Review System designed for use by medical monitors, clinical research associates, managers and other nontraditional users on the clinical staff. Written using Version 5.18 SAS/AF software, CLINACCESS has the following capabilities: single or double-key data entry; viewing and querying of data; graphics; descriptive statistics; and report generation. CLINACCESS features an extensive context sensitive help system and an online tutorial to aid the new or infrequent user. REMOTE DATA ENTRY AND MONITORING Use by investigators Figure 3 shows the main CLINAccess menu. Users select one of the options by entering its number on the "Select Option" line and pressing enter. The Database Administrator is additionally permitted to structure new datasets, paint data entry screens, and manage study libraries. A key feature of CLINACCESS is its data dictionary which is called the Clinical Questions Catalog (CQC). The Clinical Questions Catalog ensures uniformity of variables across studies, facilitating the pooling of information. The CQC also simplifies the study definition process. To create a new dataset, the Database Administrator simply places an X in the selection field for any variable which is to be included in the new dataset. All the standard variable attributes (format, informat, label, type, and length) are automatically defined. To provide flexibility while enforcing standardization, the label, informal, and I DBMS I STATISTICAL ANALYSIS I AUTOMATED TABLE AND GRAPH GENERATION CLINICAL DATA REVIEW Use by in-house medical staff I CANDA Use by FDA Reviewers Figure 2: Future CIS 22 ROSENBERG I Clinical Info Systems nAc c e s s C (tm) Select Option ===> +-------------------- SELECT AN OPTION --------------------+ 5 Descriptive statist;cs Enter data 6 Written reports 2 View a dataset 3 Rearrange data 7 Learn to use ClinAccess 4 Graphics 8 Exit Cl inAccess +----------------------------------------------------------+ PF1 HELP Level 1 (Main Menu> ENTER to select Figure 3: The CuNAccEss Main Menu 4, the user specifies the study and dataset for the report. If the user is unsure of the dataset, he or she can check a box and a list of available datasets appears (Figure 5). In Figure 6, the user selects whether all variables are to be displayed in the report or only selected variables. format may be customized to the study, while the name, type, and length of the variable are fixed. CuNACCEss's strength is the ease with which data can be accessed and manipulated. For example, Figures 48 illustrate the process of creating a report. In Figure DATA LIST REPORT C011111and ===> +--·--------------- STUDY AND DATASET NAME -----------------+ 5274 studY x_ Check (X) here and press enter to see a list of datasets for the above study. PF1 HELP PF6 CANCEL PF3 CONTINUE Figure 4: Selecting a Study and Dataset 23 ROSENBERG I Clinical Info Systems DATA LIST REPORT Conmand ===> +··············· ··· STUDY AND DATASET NAME ················· + S274 study ---------------- ---· List of Members In SAS Library------- ---------· COnllland ===> Libname: S274 MASTER _KEYS LAB ADV_RX CCMED Figure 5: A List of Datasets is Displayed In this case the user has decided to select which variables will be included in the report. Note also that CLINACCESS has selected the variables ID and WEEK for automatic display. These variables will be used to clarify the report. In Figure 7, the user selects which variables will appear in the report by placing an X next DATA LIST REPORT C011111and ===> I I to each variable to be included. To print sums in the report, the user additionally checks the sum column. Figure 8 shows the finished report. The data is automatically displayed by identil)'ing variables, in this case the patient ID and the week of the visit. +--------------- ---------------- ---------------- -------------+ I I STUDY AND DATASET NAME: S274.LAB +······------· VARIABLES AUTOMATICALLY DISPLAYED ············+ ID WEEK +··············· · OTHER VARIABLES TO DISPLAY ----············+ Choose one: __ Display all other variables in the dataset. X_ Select other variables to display from a List. +··············· ··----· OPTIONAL TITLES ················· ····+ I I +--------------- ---------------- ---------------- -------------+ PF1 HELP PF5 variables PF6 CANCEL PF9 ZOOM PF3 SUBMIT Figure 6: The User Fills in Blanks on the Report Program Screen 24 ROSENBERG I Clinical Info Systems Screen Obs Edit dataset: S274.LAB C011111and ===> 3, ••• to specify Type an X in the DISPLAY colU1111 or use 1, 2, the order displayed. Use X to indicate which variables to SUM. DESCRIPTION TYPE DISPLAY SUM NAME CALCIUM NUM X NUM SGOT X X NUM SGPT X X GLUCOSE NUM NUM IRON NUM PHOS NUM BUN -X BILIRUBN NUM CREATNIN NUM ALBUMIN NUM NUM SODIUM Phosphorus Bilirubin Creatinine PF7 BACKWARD PF1 HELP PF3 SUBMIT PF8 FORIIARD Figure 7: Selecting Variables to Display and Sum DATA LISTING FOR STUDY S274 DATASET IS LAB 10 101 IIEEK 0 4 8 12 16 20 BILIRUBIN CALCIUM 0.6 0.8 0.9 0.6 0.8 0.6 9.5 9.8 10.1 10.1 9.7 9.6 1.0 0.7 1.0 0.9 0.7 0.9 0.8 9.0 9.2 9.6 9.4 9.2 9.7 9.5 101 102 N= SGPT 13 8 12 10 12 12 13 14 14 17 15 19 ··---------92 -----------67 6 N= 102 SGOT 0 4 8 12 16 20 28 17 12 11 10 13 14 11 -----------88 7 16 10 13 11 15 17 14 -----------96 April 6, 1989 Figure 8: The Completed Report mainframes under either the MVS or VM/CMS operating systems. CLINACCESS is an example of the kind of applications which can be developed using Version 5 SAS software. CLINACCESS Version 5 is available on IBM 25 ROSENBERG I Clinical Info Systems VERSION 6 ENHANCEMENTS opened for either data entry or browsing. The windows can be resized or zoomed to fill the entire screen. The introduction of SAS/AF in Version 5 extended the power of SAS software to the non-traditional user. Version 6 further extends that power with greater ease of use and the addition of many desirable features associated with relational database management systems (RDBMS). These enhancements combine to transform SAS into a connectivity tool which spans hardware and software platforms as well as databases. Datasets can also be linked using SCL. One thorny problem in clinical data management is to display comments made by investigators. A solution is to place the comments in a separate dataset. An SCL link can take a reviewer from an observation stored in one dataset to a comment for that observation stored in the comment dataset. When finished reading the comment, a single keystroke returns the user to the dataset he was browsing. The Version 6 enhancements include: Screen Control Language available in SAS/AF and SAS/FSP software. • Host windowing environments PROC SQL which adds the emerging standard for fourth generation relational database query languages to SAS. • PROC ACCESS and Multiple Engine Architecture which provide transparent access to databases such as Oracle, DB2, SQL/DS, and Rdb/VMS. • Multi-Vendor Architecture, which permits applications to run on multiple hardware platforms. • Indexing of SAS datasets, which provides interactive applications with faster access to data. Compressed SAS datasets, which can save disk storage space. The other Version 6 enhancements will be available in Version 6.06 or subsequent releases of SAS. Version 6.06 will initially be available for IBM mainframes under MVS or VM/CMS, DEC VAX computers under VMS, and PC compatibles under OS/2. SCL will be substantially enhanced in Version 6.06, including the addition of further database management functions, as well as support for host windowing environments. These enhancements will support a common user interface with pull down menus, dialog boxes, and other point and shoot tools. In particular, under the OS/2 Presentation Manager and DEC Windows, graphical user interfaces are supported, providing for the first time, the power of SAS software with the ease of use of a Macintosh like interface. · Version 6.03 of SAS is currently available on IBM compatible PC's under the MS-DOS operating system and on selected HP and Sun Microsystems computers under UNIX. This release includes Screen Control Language (SCL), a true programming language which generates stored object code. In SAS/AF software, SCL is specifically designed to create interactive fullscreen applications. In SAS/FSP software, SCL performs sophisticated interactive edit checks during data entry. The syntax of SCL is similar to that of the SAS DATA step, so experienced SAS programmers will quickly feel comfortable. Another addition to Version 6.06 is Multiple Engine Architecture. Multiple Engine Architecture provides the ability to use data stored in data structures other than a SAS dataset, for example data stored in another database management system (DBMS). Currently, interfaces between SAS and a DBMS are constructed as in Figure 13. A program is used to identify the data available in the DBMS. The user selects the variables and/or records desired and these are extracted from the DBMS and restructured in the form of a SAS dataset. This extra step of extracting data from a database and creating a SAS dataset can be a considerable obstacle to the non-traditional user. In addition, since the data is physically removed from the DBMS, as new data are added or corrections are made to the DBMS, the extracted data ceases to be current and must be reextracted. Version 6.03 has also added useful database management features such as the ability to display information from several datasets simultaneously. Customized screens resembling case report forms can be developed which display data from more than one dataset, for example demographics and adverse events. Data entry can now be performed either in CRF or in table view. Additionally, in table view, multiple windows can be Under multiple engine architecture, SAS applications will be able to run directly against other databases. A 26 ROSENBERG I Clinical Info Systems pharmaceutical industry where companies may use several types of computers, e.g. a corporate mainframe, a departmental mini-computer such as a VAX, and intelligent workstations or PC's. Because the CIS will have a common user interface across the hardware platforms, training costs can be minimized. Extract Program User Specifies Variables PROC SQL and indexing bring to SAS two important database management features for performing queries. SQL is the emerging standard for relational database query languages. The SAS implementation will permit queries of both SAS datasets and other database structures, through multiple engine architecture. Indexing is a common database management feature that can speed on-line queries. Finally, compressed datasets will reduce storage costs. SAS Dataset Created Data Used by SAS Application Figure 13: Current Extract Process SUMMARY new procedure, PROC ACCESS, is used by the database administrator, to define the mapping between the host DBMS structure and SAS. Thereafter, access to the DBMS is transparent to the user. As shown in Figure 14, a SAS program that requires data sends a request to the engine supervisor, a portion of the SAS system. The engine supervisor selects the appropriate software engine to read the data, and the information is provided to the requesting program. No SAS dataset needs to be created. SAS Application Requests Data I Engine As the role of the Clinical Information System expands to encompass non-traditional users such as investigators, medical monitors, CRA's, and FDA reviewers, pharmaceutical companies are faced with an increasingly complex web of technology. In an effort to simplify the process, we might look for ways to integrate the various CIS processes. One way to do this is to explore existing standards in an effort to expand their use and leverage the investment already made in training personnel and incorporating the technology. SAS software is the de facto standard in the pharmaceutical industry for performing statistical analyses and presenting the results in graphical or tabular forms. Once data is in machine readable form, most of the subsequent processing is typically performed in SAS. Consequently, it is reasonable to examine whether SAS can play an expanded role in the CIS. r<~ I Supervisor I I I Although initially a statistical package, much of the recent development of SAS has been to add features commonly associated with relational database management systems and fourth generation languages. Version 6 of SAS adds a number of features that are of particular strategic importance to the pharmaceutical industry. Version 6 offers the potential to easily port CIS applications to other hardware platforms due to multi-vendor architecture. A CIS might operate on personal workstations, department VAX computers, and enterprise wide IBM mainframes, all with a Proper Engine I I Figure 14: Multiple Engine Architecture Multi-vendor architecture refers to the ability of a SAS program written for one computer to be used on other computers. This is particularly important in the 27 ROSENBERG I Clinical Info Systems common user interface. The addition of PROC SQL, indexing, data compression, and other database management tools has the potential to make storage of data in SAS datasets sufficiently powerful for some companies. Other companies that require a database management system can develop applications independent of the particular DBMS used, due to multiple engine architecture. The transition from one DBMS to another or the sharing of information between divisions that use different databases can be enhanced. Wallace, Emily P. (1989). Database Interfaces Under the Version 6 Engine Architecture. Proceedings of the Fourteenth Annual SAS Users Group International Conference. SAS Institute Inc., cary, NC. pp. 347-349. ACKNOWLEDGEMENTS CLINACCESS is a trademark of MAJARO lNFOSYS1EMS, INC., Mountain View, CA, USA All CLINACCESS screens shown are Copyrighted (C) 1988, 1989 by MAJARO INFOSYS1EMS, INC. and are used by permission. This paper has explored many of the existing and emerging features of SAS software and has shown that it may be able to play a prominent role in developing integrated, powerful, yet easy to use clinical information systems for the 1990's. Many of the ideas of this paper have already been incorporated into the CuNACCEss™ clinical data review system. CuNACCESS is available on IBM mainframes under MVS and VM/CMS and is currently being ported to IBM PC compatibles under MS-DOS. Support for other hardware platforms and operating systems is anticipated. SAS, SAS/AF, and SAS/FSP are the registered trademarks of SAS Institute Inc., cary, NC. DEC, VAX, VMS, and Rdb/VMS are trademarks of Digital Equipment Corporation. Macintosh is a registered trademark of Apple Computer Inc. IBM is a registered trademark and DB2, SQL/DS, OS/2, and Presentation Manager are trademarks of International . Business Machines Corporation. Oracle is a registered trademark of Oracle Corporation. MS-DOS is a registered trademark of Microsoft Corporation. UNIX is a registered trademark of AT&T. For a number of years now, we've witnessed the evolution of SAS software into a product with greater interactivity and data management capabilities. Version 6 is a major step in that evolution. Companies that start now to explore these new capabilities and learn to exploit them, will have the potential of realizing substantial advantages over their competitors in terms of reducing the cost and time needed to bring a new drug to the market. MAJARO INFOSYS1EMS INC. provides statistical and information management services to the pharmaceutical, biotechnology, and food products industries, and specializes in extending computer technology to non-traditional users. REFERENCES Rosenberg, Martin J. (1988). Using the SAS System to Facilitate Clinical Trials Research and NDA Approval. Proceedings of the Thirteenth Annual SAS Users Group International Conference. SAS Institute Inc., cary, NC. pp. 550-556. For further information regarding this paper, please contact: Martin J. Rosenberg, Ph.D. MAJARO INFOSYS1EMS, INC. 99 East Middlefield Road Suite 31 Mountain View, CA 94043 tel. (415) 961-8432 (415) 961-9260 Rosenberg, Martin J. (1989). An Integrated Approach to Computer Systems for NDA Preparation and Presentation. Proceedings of the Fourteenth Annual SAS Users Group International Conference. SAS Institute Inc., cary, NC. pp. 786-792. 28