* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Role of buffalo in the maintenance of fmdv,
Survey
Document related concepts
Transcript
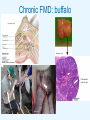
Role of buffalo in the maintenance of foot-and-mouth disease virus Bryan Charleston Study design 3 KNP buffalo isolates 6 month old Nguni cattle (n = 4) SAT1/KNP/196/91 SAT2/KNP/19/89 SAT3/KNP/1/08/3 PK cells SAT-3 OVI cattle titration ≈ 1 × 104 TCID50 SAT-1 SAT-2 Skukuza buffalo challenge study ≈ 5 × 105 TCID50 Necropsy N = 4 buffalo Necropsy Necropsy N = 4 buffalo N = 4 buffalo 35DPC 95DPC 156DPC 163DPC 185DPC 1.25IU/kg N = 4♀ Institute for Animal Health 400DPC 1.25IU/kg ACTH stimulation (Synacthen®) N = 4 buffalo N = 16 FMD free buffalo (7♂9♀) 10 – 30 months old Imfolozi Game Reserve, KZN 299DPC Necropsy N = 4 buffalo Day 302 Progesterone IUD Estrumate Acute FMD: naïve buffalo 1DPC 2DPC Acute FMD: naïve buffalo Conclusion • SAT co-infection in cattle – severe clinical FMD • 10 × dose in naïve buffalo: • mild clinical FMD • integrin αvβ6 expression in tissues typically associated with FMD lesions • viraemic for at least 5 to 7 days • No leucopenia • High levels type 1 IFN • seroconverted to all three serotypes by day 14 3D primer and probe SAT1 primer and probe SAT2 primer and probe SAT3 primer and probe Chronic FMD: buffalo Casteleyn et al., Budras, Bovine Anatomy Follicles with central crypt M Hofmeyr Institute for Animal Health Chronic FMD: buffalo Virus isolation (IBRS cells) Number of buffalo 8 Right tonsil swab Probang 7 6 5 4 3 2 1 0 50 DPC *109 DPC 35 35 *One round VI only () = passage number on IBRS cells Institute for Animal Health 126 DPC 136 DPC 155 DPC 162 DPC 168 DPC 172 DPC 185 DPC Day post challenge Chronic FMD: buffalo 3D primer and probe SAT1 primer and probe SAT2 primer and probe SAT3 primer and probe Institute for Animal Health Chronic FMD: buffalo 95DPC 10µm 100µm 100µm Anti-3B (D5) IB11 Merge 10µm Institute for Animal Health FMD: buffalo Discussion points • Despite close contact, +ve virus isolation and ACTH stim – no transmission/ seroconversion in cattle! • Consistent with previous reports: • no transmission/ transmission only after months of contact • Rx buffalo dexamethasone (Gainaru et al., 1986) • Rx cattle dexamethasone for 3 weeks (Ilott et al., 1997) – inhibited shedding of FMDV • Co-infection cattle rinderpest/ bovine herpes 1 viruses (McVicar et al., 1977) – no increase virus recovery/ transmission • Pattern of transmission from captive buffalo during acute infection is also variable • same pen: buffalo to buffalo/ buffalo to cattle× (Gainaru et al., 1986)/ buffalo to cattle× (Dawe et al., 1994) • adjoining pen: buffalo to impala× (Gainaru et al., 1986) Knowledge gaps and questions • Improved methods to isolate virus/ viral sequences from carrier buffalo • Indentify sites of virus localisation and replication in buffalo • Propose hypothesise for mechanisms of persistence • Test potential “triggers” for transmission: short term stress? Long term stress? “childhood epizootic”? • How infectious are buffalo during acute infection? Virus excretion compared cattle/sheep/pigs. Transmission despite no/ limited FMD lesions? Institute for Animal Health Future work • FMDV maintains high force of infection in buffalo herds > 98% buffalo in KNP exposed to all three SAT serotypes by age 2 (Thomson, 1992) High rate of infection suggests common mechanism for inter-annual perpetuation Avoids auto-extinction small isolated herds • Crucial that we understand how FMDV persists in isolated buffalo herds Primary objective to identify triggers to affect transmission from carriers. If we can’t even understand this - how understand in livestock, large populations, global, multiple serotypes? • 3 year longitudinal study of isolated breeding herd in KNP – – – – – • Account for environmental factors absent from experimental studies Identify factors affecting FMDV status (age, sex, seasonal, co-infections etc.) Phylogenetic and antigenic phenotype analysis of FMDV isolates Establish infection parameters in buffalo calves (naïve population) Test triggers under experimental conditions We hypothesise: – Previous demonstration of FMDV particles in lymph tissue on germinal centre FDCs provides a means of delayed viral clearance (extracellular reservoir) • To affect transmission: – Immune stimulation (enhance immune-complexed FMDV replication in Fc receptor expressing cells) – Or suppress antibody response – for example protein-calorie restricted diet (Watson, 1985) Acknowledgments Nick Juleff Lin-Mari de Klerk Lorist Roy Bengis Louis van Schalkwyk At Dekker Scalk van Dyk Dave Cooper Francois Maree Ivan Morrison Berry Mutowembwa Dan Haydon Brenda Botha Livio Heath Katherine Scott Pamela Opperman Belinda Blignaut Ferran JORI, Cirad Mammal Research Institute University of Pretoria Markus Hofmeyr Peter Buss Jenny Joubert