* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download MS PowerPoint - Catalysis Eprints database
Transition state theory wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Marcus theory wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
History of electrochemistry wikipedia , lookup
Theory of solar cells wikipedia , lookup
Nickel–cadmium battery wikipedia , lookup
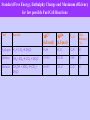
Frontiers in Electrochemistry S. Chandravathanam, Research Scholar National Centre for Catalysis Research Department of Chemistry Indian Institute of Technology, Madras Orientation Programme in Catalysis for Research Scholars, 2008, 1/12/08 Contents Fundamentals Frontier Applications of Electrichemistry Batteries Fuel Cells Supercapacitors Photoelectrochemical cells What is Electrochemistry? - all about the study of Electrified Interfaces and its consequences. What is an Electrified Interface? It is the two dimentional geometrical boundary surface separating the two phases. What is an Electrified Interphase? It is the three dimensional region of contact between the two phases in contact at their boundary. Electrified Interface Electrified Interface Whenever an uncharged metal or electron conductor contacts with an ionic solution manifests an excess surface electric charge on both sides of the interphase; - Creates a gigavolt per meter (107 V/cm)field in the interface region, with the electroneutrality of the bulk metal. The effect of this enormous field at the electrode interface is the essence of electrochemistry. Examples of Electrified Interfaces Why do Colloidal particles move under electric fields? The electrified interface between the Colloidal particle and the medium causes a potential difference in the interface, which interacts with the externally applied electric field lies the basis for coating of metals. Is the friction between two solids in presence of liquid film an Electrified interface? Yes the efficiency of a wetted rock drill depends on the double layer structure at the metal/drill/aqueous solution interface. The mechanism by which a nerves carry messages from brain to muscles is based on the potential difference across the membrane that separates a nerve cell from the environment. What is an Electrochemical reaction? - it is the chemical transformation involving the transfer of electrons across an interface. Examples are, 2H+ + 2e H2 C2H4 + 4 H2O 2 CO2 + 12 H+ + 12 e Remarkable distinction from the chemical reaction is the controlled way in which a chemical substance produce another substance. Batteries What are Batteries? - Electrical Energy Storage Device - Store the electricity produced else where by driving the charging reaction through the free energy hill by splitting the reaction in two parts, each to take place on each electrode. - as soon the electrodes are connected the charged reactants are ready to react together to down the free energy hill by discharging the electricity. HISTORY OF BATTERIES 1800 1836 1859 1868 1888 1899 Voltaic pile: silver zinc Daniell cell: copper zinc Planté: rechargeable lead-acid cell Leclanché: carbon zinc wet cell Gassner: carbon zinc dry cell Junger: nickel cadmium cell HISTORY OF BATTERIES 1946 1960s 1970s 1990 1991 1992 1999 Neumann: sealed NiCd Alkaline, rechargeable NiCd Lithium, sealed lead acid Nickel metal hydride (NiMH) Lithium ion Rechargeable alkaline Lithium ion polymer Battery Types Non-Chargeble (Disposable) Batteries - Primary Chargeble Batteries - Secondary Primary (Disposable) Batteries Leclanché Cells (zinc carbon or dry cell) Alkaline Cells Mercury Oxide Cells Zinc/MnO2 Cells Aluminum / Air Cells Lithium Cells Liquid cathode lithium cells Solid cathode lithium cells Solid electrolyte lithium cells Lithium-Iron Cells Magnesium-Copper Chloride Reserve Cells Secondary (Rechargeable) Batteries Lead–acid Cells Zinc/MnO2 Cells (Mechanical Recharging) Nickel/Cadmium Cells Nickel/Metal Hydride (NiMH) Cells Lithium Ion Cells Rechargeable Alkaline Manganese Cells Alkaline Cells Half cell reactions Zn + 2 OH- —> ZnO + H2O + 2 e2 MnO2 + H2O + 2 e- —>Mn2O3 + 2 OH- The overall reaction Zn + 2MnO2 —> ZnO + Mn2O3 E = 1.5 V Applications: Radios, toys, photo-flash applications, watches Storage density about twice that of the carbon-zinc cell, but more expensive Lead–acid Cells Applications: Motive power in cars, trucks, standby/backup systems Can be recharged hundreds of times and very cheap, but bulky and environmentally noxious Zinc/Air Cells Anode: Amalgamated zinc powder Cathode: Oxygen (O2) Electrolyte: Potassium hydroxide (KOH) Half-reactions: Zn + 2OH- —> Zn(OH)2 1/2 O2 + H2O + 2e —> 2 OHOverall reaction: 2Zn +O2 + 2H2O —> 2Zn(OH)2 E = 1.65 V Applications: Hearing aids, pagers, electric vehicles Lithium ion Cells Charging Discharging Anode: lithium ions in the carbon material Cathode: lithium ions in the layered material (lithium compound) Anode Li1-XCoO2+ CnLix LiCoO2 + Cn Cathode LiCoO2+ Cn Li1-XCoO2 + CnLix The lithium ion moves from the anode to the cathode during discharge and from the cathode to the anode when charging. Applications: Laptops, cellular phones, electric vehicles Charge/discharge curve for Lead – acid Battery Real time graph charging the Pb-acid battery battery Echarge = Erev + + IR Edischarge = Erev - - IR Behavior of the battery at different discharging rate Pb-acid battery; 100 mA (1), 200 mA (2) and 300 mA (3) Comparison of some Batteries Battery Type Specific Energy (Wh/kg) Specific Power (W/kg) Life Cycles Application Lead acid 35 – 40 180 300 - 400 as a Booster power for start-up in internal combustion engine Nickel cadmium 45 - 55 150 700 - 1200 Toys Zn - MnO2 8 - 64 25 Most of solid state devices like hearing aid, flash light batteries, portable TV, computer, etc. Zn - Air 200 30 Mechanically Automative application rechargable Ni - MH 150-200 250-1000 700 – 1200 Automative application Li ion 100-200 400-1200 Laptops, cell phones Fuel Cells History of Fuel Cells Discovery – Sir William Grove – a British Judge (1839) Sir William Grove Rediscovery – Francis Thomas Bacon – an Engineer working in a turbine Company (1932) – behind NASA’s use of fuel cells in space flights (as auxillary power source for low weight/ unit of energy) Francis Thomas Bacon W.R. Grove, On Voltaic Series and the Combination of Gases by Platinum; Phil. Mag. XIV, 127-130 (1839) What are Fuel Cells? - are energy conversion devices, convert the free energy change of a chemical reaction directly into electricity (electrochemical energy conversion) and not as heat in a chemical reaction. Comparison of Fuel Cells with Internal Combustion Engines ICE-2 Thermal Energy Mechanical Energy ICE-3 ICE-1 Chemical energy of fuels Fuel Cell Electrical Energy Schematic of energy conversion in Fuel cells and Internal Combustion Engines (ICE) Comparison of Batteries and Fuel Cells Batteries – Energy Storers (Utilize the electricity produced else where to drive the charging reaction through the free energy hill). (Effectiveness of batteries encompasses situations where it would be impractical to store fuel to make electricity on the spot, for example in portable equipments like telephones, tape recorder etc.) Fuel Cells – Energy generators ( Electricity is generated as a result of spontaneous chemical reaction spilt into two half reactions) Types of Fuel Cells - Space application - Transportation applications - high volumetric energy density - avoids the need of pure H2 - envisaged for stationary power plants Fuel Cell Efficiency -G = Wrev - PV Wrev – Useful work PV – Work of expansion In Fuel cells, no moving parts andso no work of expansion -G = Wrev For an electrochemical reaction, Overall reaction 2H2 4H+ +4e O2 +4H+ + 4e 2H2O 2H2 + O2 2H2O The electrical work in transporting these 4e across the potential difference Ve, = 4FVe Ve – thermodynamic equillibrium potential of the reaction -G = 4FVe For an n electron transport, -G = nFVe Maximum amount of useful electrical work obtainable from a chemical reaction or Intrincically available electrical work of a chemical reaction But H is the total energy change of the reaction, including the the entrophy change for ordering and disordering of reactants and products. Efficiency of electrochemical energy conversion = G / H = -nFVe / H is not 100% efficient. But has the theoretical maximum of 90%; But heat engine has the theoretical maximum of 25 – 40 %, based on the workable temperature range. Efficiency of heat engine = (T1 – T2) / T1 Performance limitations of Fuel Cells Activation polarization Ohmic polarization Mass-transport polarization Current-Potential curve for H2 - Air fuel cell at 80 °C the practical obtainable maximum energy conversion efficiency ~ 65% ( 2 times that of heat engine) Why Pt ? > go. > 0 > go. > 0 H - 0 H - 1 M – H Bond Strength, KJ /mol Relationship between current densities for hydrogen evolution and M – H Bond Strength 13 Standard Free Energy, Enthalphy Change and Maximum efficiency for few possible Fuel Cell Reactions Fuel Reaction Hydrogen H2 + ½ O2 2H2O Ve (V) -G° -H (kJ/mol) (kJ/mol) Max. Efficiency (%) 56.69 68.32 1.229 83 Methane CH4 + 2O2 CO2 + 2H2O 195.50 212.80 1.060 92 Methanol CH3OH + 3/2O2 CO2 + 2H2O 168.95 182.61 1.222 93 Advantages of Fuel Cells Higher intrinsic efficiency Lesser CO2 accumulation in the atmosphere Second Fuel Cell Principle – Electroregenerative Synthesis of materials Advantage - energy production is the by-product Eg., Electroregenerative synthesis of dichloroethylene Anode reaction, C2H2 + 2Cl- C2H4Cl2 + 2e Cathode reaction, Cl2 + 2e 2Cl- Super Capacitors What are Supercapacitors? - are the electrochemical storage devices, storing electricity in the form of Electrochemical double layer. - different from batteries (elctricity stored as chemical), or dielectric capacitors or parallel plate condensors (electricity is stored electrostatically in a dielectric material between two metal plates). Models of the Double Layer Structure of Electrified Interface a) Helmholtz model b) Gouy-Chapman model of the diffuse layer c) Stern's model, combining (a) and (b) Types of Capacitors Schematic of different ways of electricity storage Comparison of Supercapacitors with Batteries Supercapacitors have very high Specific Power of 102 kW/Kg (100 - 1000 times higher than batteries), uncomparable cycle life of 105, less Specific Energy ( 40 Wh/Kg) store and deliver electricity by electrostatic charging takes place at the two dimensional interface without any irreversible or slow chemical phase change, exhibit fast charging and longer cycle life. no serious disposal and safety hazard Ragone plot for various energy storage and conversion devices Ragone plot showing energy density vs. power density for various devices along with discharge time. Capacitance of the Capacitors The capacity of the parallel plate condensor C (in farads or coulombs per volt) = A ε / 4 π d A- Area of the contact plates d- distance between the plates ε – dielectric constant of the medium between the plates The relation between capacitance "C" and the inter-plate voltage "V" arises from accumulation of a charge "q“ is, C = q/V or q = CV Capacitance of the Double-layer Capacitor The charge density "q" (coulomb/cm2) of electrons and ions at the interface is dependent on the potential difference, ΔΦ, across this double layer so that a differential capacitance "Cdl" arises, is determined by, Cdl = dq/d(ΔΦ) or Δq/ΔΦ The difference of potential extends beyond the immediate layer of solvated ions in the compact, capacitor-like (Helmholtz) region, out into solution, so that a further diffuse-layer capacitance "Cdiff" arises. It combines with the capacitance of Helmholtz region "CH" in series, electrically, so that, 1 — = Cdl 1 1 — +— CH Cdiff Applications of Supercapacitance Booster for hybrid vehicles with fuel cell or battery during start-up or acceleration. Regenerative braking can be used to charge the Supercapacitor for its fast charging rate. Pseudocapacitance - Double-layer capacitance "C" or "Cdl" is non-faradaic or electrostatic . Pseudocapacitance "CΦ“ is faradaic (Capacitance due to charge transfer process) - when the extent of faradaically admitted charge "q" depends linearly, or approximately linearly, on the applied voltage "V". For such a situation, there is a mathematical derivative, dq/dV that would be constant, which is equivalent to, and measurable as, a capacitance. - The pseudocapacitance can increase the capacitance of an electrochemical capacitor by as much as an order of magnitude over that of the double-layer capacitance. Pseudocapacitance of RuO2 cyclic voltammetry behavior of a reversibly chargeable electrochemical capacitor material RuO2 Limitation of Capacitance of Double layer Capacitor - charging of the high-area, porous-electrode structures that are required for achieving large capacitance densities (farads/g) encounters limitations of rate due to the distributed electrolytic and contact resistances within the pore structure of such materials. Photoelectrochemical Cells What is Photoelectrochemistry? - Generation of current following the exposure of Semiconductor electrodes to electromagnetic radiation. Generation of bands in solids from atomic orbitals of isolated atoms - metals do not absorb solar radiation. - insulators also cannot absorb as the band gap is so high (> 5 eV), the energy of the solar radiation is not sufficient to excite electron from valence band (VB) to the conduction band (CB). - Semiconductors have the band gap not as large, promotion of electron is possible with the solar radiation. Schematic diagram of the energy levels of an a) intrinsic semiconductor, b) an n-type semiconductor and c) a p-type semiconductor (a) - Charge carriers in Semiconductor can be altered by doping. (b) (c) - Addition of Group V element (P. As) into Group IV element (Si, Ge) introduces occupied energy levels into the band gap close to the lower edge of CB, thereby allowing facile promotion of electrons into the CB (n-type Si, or n-type Ge; majority charge carriers - e). - Addition of Group III elements (Al. Ga) into Group IV elements introduces vacant energy levels into the band gap close to the upper edge of the VB, which allows the facile promotion of e from the VB (p-type Si, or ptype Ge; majarity charge carrier - holes). Fermi level is defined as the energy level at which the probability of occupation by an electron is ½; - for an instrinsic semiconductor the Fermi level lies at the mid- point of the band gap. - Doping changes the distribution of electrons within the solid, and hence changes the Fermi level. - For a n-type semiconductor, the Fermi level lies just below the conduction band, whereas for a p-type semiconductor it lies just above the valence band. - In addition to doping, as with metal electrodes, the Fermi level of a semiconductor electrode varies with the applied potential; for example, moving to more negative potentials will raise the Fermi level. Model of the Semiconductor-Electrolyte interphase Metal-Electrolyte Interface Idealized interface between a semiconductor electrode / electrolyte solution. If the redox potential of the solution and the Fermi level do not lie at the same energy, movement of charge between the semiconductor and the solution takes place in order to equilibrate the two phases. Excess charge located on the semiconductor does not lie at the surface as it would for a metallic electrode, but extends into the electrode for a significant distance (100-10,000 Å) - space charge region. Hence, there are two double layers to consider: the interfacial (electrode/electrolyte) double layer, and the space charge double layer. For an n-type semiconductor electrode at open circuit, the Fermi level is higher than the redox potential of the electrolyte, hence electrons will be transferred from the electrode into the solution positive charge associated with the space charge region, and is reflected in an upward bending of the band edges as majority charge carrier is removed from this region, this region is referred to as a depletion layer. Band bending for an ntype emiconductor (a) and a p-type semiconductor b) in equilibrium with an electrolyte For a p-type semiconductor, the Fermi layer is lower than the redox potential, hence electrons must transfer from the solution to the electrode generates negative charge in the space charge region, causes a downward bending in the band edges. Since the holes in the space charge region are removed by this process, this region is again a depletion layer. Mechanism of production of photocurrent by an p-type photocathode Mechanism of production of photocurrent by an n-type photoanode Intensity of Solar Energy Absorbtion by Semiconductors of different band gaps energies - low band gap materials absorb more of solar radiation, but are easily photodegradable. Applications of Photoelectrochemistry Substitution of gasoline and natural gas by H2 produced from photoelectrochemical splitting of water; Solving CO2 build-up. Carrying out commercially important organic reactions (e.g., oxidation of toxic wastes, Kolbe-reaction, etc.) Summary Electrochemistry leads to the sustainable Future Energy Technologies (production, Storage, Conversion and Application) . References 1. Modern Aspects of Electrochemistry, J. O’M. Bockris and A. K. N. Reddy, Kluwer Academic, 2000. 2. Electrochemistry, Prof. B. Viswanathan et al., S.Viswanathan Publishers, 2007 3. Electrochemistry of Semiconductors, Adrian W. Bott, Current Separations 17 (1998) 87 – 91. 4. Electrochemical capacitors, Brian E. Conway, http://electrochem.cwru.edu/ed/encycl Thank You