* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Electrical properties
Survey
Document related concepts
Transcript
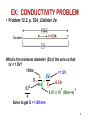
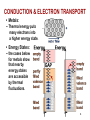
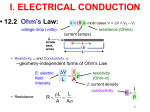
CHAPTER 12: ELECTRICAL PROPERTIES ISSUES TO ADDRESS... • How are electrical conductance and resistance characterized? • What are the physical phenomena that distinguish conductors, semiconductors, and insulators? • For metals, how is conductivity affected by imperfections, T, and deformation? • For semiconductors, how is conductivity affected by impurities (doping) and T? 1 Chapter 12 – Electrical Properties • Motivation – Why would you care about understanding how materials respond to applied electric fields? VIEW OF AN INTEGRATED CIRCUIT • Scanning electron microscope images of an IC: Al Si (doped) (d) (a) 45m 0.5mm • A dot map showing location of Si (a semiconductor): --Si shows up as light regions. (b) • A dot map showing location of Al (a conductor): --Al shows up as light regions. Fig. (d) from Fig. 18.25, Callister 6e. (Fig. 18.25 is courtesy Nick Gonzales, National Semiconductor Corp., West Jordan, UT.) (c) Fig. (a), (b), (c) from Fig. 18.0, Callister 6e. 2 Chapter 12 – Electrical Properties • Introduction – Electrical conduction – The next several slides you have seen before (Physics) – Ohm’s law – relation between current I (time rate of charge passage) and the applied voltage V IR V []Volts( J / C ) I [] Amperes(C / s) R[]ohms(V / A) • R – resistance, influenced by specimen configuration, often independent of current • Resistivity r r RA l r VA , r[] m lI A – cross sectional area perpendicular to current l – distance between points where V is measured Chapter 12 – Electrical Properties • Introduction – Electrical conductivity – Typically the electrical conductivity is used to specify the electrical properties of materials 1 r , [] m 1 • Conductivity describes the ease with which a material is capable of conducting an electrical current • Can also express Ohm’s law as ξ is field intensity, J is the current density, J = σξ; ξ = V/ l and l is the distance between points where V is applied Amazing point – solids exhibit a range of electrical conductivities spanning over 27 orders of magnitude! ELECTRICAL CONDUCTION • Ohm's Law: DV = I R voltage drop (volts) current (amps) resistance (Ohms) • Resistivity, r and Conductivity, : --geometry-independent forms of Ohm's Law E: electric field intensity • Resistance: R DV I r L A rL L A A resistivity (Ohm-m) J: current density conductivity I r 3 CONDUCTIVITY: COMPARISON • Room T values (Ohm-m) -1 Selected values from Tables 18.1, 18.2, and 18.3, Callister 6e. 4 EX: CONDUCTIVITY PROBLEM • Problem 12.2, p. 524, Callister 2e: What is the minimum diameter (D) of the wire so that DV < 1.5V? 100m < 1.5V L DV R 2.5A A I D2 7 -1 6.07 x 10 (Ohm-m) 4 Solve to get D > 1.88 mm 5 Chapter 12 – Electrical Properties • Typically materials fall into three categories – Conductors – metals are good conductors ( ~ 107 (-m)-1) – Insulators – poor conductors ( ~ 10-10 – 10-20 (-m)-1) – Semiconductors – in the middle ( ~ 10-6 – 104 (-m)-1) • Electronic/ionic conduction – Electric current results from the motion of electrically charged particles due to forces acting on them from an externally applied electric field – Positive charges – accelerated in field direction, negatively charges – accelerated in opposite direction – In most solids a current arises from the flow of electrons (electronic conduction) – In ionic solids – a net motion of ions is possible that can produce a current (ionic conduction) Chapter 12 – Electrical Properties • Band structures in solids – You are used to thinking of electron energy states as being very well defined (molecular orbital theory) – For solids containing larger numbers of atoms this becomes blurred This is what happens when 12 atoms approach one another (note r is the x-axis) Electron energies start to split (this is due to interactions between atoms) This is only relevant when the atoms come close together (i.e. bonded) CONDUCTION & ELECTRON TRANSPORT • Metals: -- Thermal energy puts many electrons into a higher energy state. • Energy States: -- the cases below for metals show that nearby energy states are accessible by thermal fluctuations. 6 Chapter 12 – Electrical Properties • Band structures in solids – Another picture -- Why does this matter? Whether or not there is an energy band gap, and if so, how large it is, determines the electrical properties of materials Why? For N atom solid s band has N states p band has 3N states Can have empty or partially filled bands Electrical properties are a consequence of the band structure! Chapter 12 – Electrical Properties • Band structures in solids – Four types of band structures are possible at 0 K Ef – Fermi level: highest filled state at 0 K The four figures above are the key point of this chapter – we’ll go through them one at a time! Nomenclature – valence band (filled states), conduction band (empty band) Band gap – electrons are not allowed to have energies in this range Chapter 12 – Electrical Properties • Band structures in solids – Band structure below – metal • Outermost band below the band gap (what is the band gap?) is only partially filled – Can see this for metals, particularly metals with one 1 s electron (e.g. Cu) – Have N copper atoms, the 4s band can accommodate 2N 4s electrons – Based on this should Cu be a good conductor? Chapter 12 – Electrical Properties • Band structures in solids – Band structure below – metal (why do you think that is?) • Overlap between an empty band and a filled band (e.g. Mg) • For Mg the 3s and 3p energy bands overlap • Why do you think this is a good conductor? Chapter 12 – Electrical Properties • Band structures in solids – Insulators/semiconductors • Both of these are similar • Well defined energy difference between the filled valence band and the empty conduction band • Large energy difference (band gap) – the material is an insulator (Large? > 2.0 eV (3.2x10-19J)) • Small (< 2.0 eV) band gap – semiconductor ENERGY STATES: INSULATORS AND SEMICONDUCTORS • Insulators: --Higher energy states not accessible due to gap. • Semiconductors: --Higher energy states separated by a smaller gap. 7 Chapter 12 – Electrical Properties • Conduction and Band Structure – Few more critical concepts • Only electrons with energies greater than the Fermi energy can be acted on/accelerated in an applied electric field • These electrons (free electrons) participate in the conduction process • Another important quantity – hole – we see what these are later, and they also participate in the conduction process (they actually have energies less than Ef, we’ll see why in a minute) – As we will see the conductive properties of materials (i.e. whether they are conductors, semiconductors, or insulators) depend on the number of free electrons and hole charge carriers Chapter 12 – Electrical Properties • Conduction and Band Structure – Metals • For electrons to become free (mobile) they must be excited into one of the empty energy states above the Fermi level • Typically this does not require much energy – Applied fields can excite large numbers of electrons into excited states • This is why metals conduct! Chapter 12 – Electrical Properties • Conduction and Band Structure – Insulators/Semiconductors • There are no longer empty states adjacent to the valence band that can be filled • Must promote electrons across the band gap (Eg) • Gap is large enough, non electrical processes are needed to excite the electrons • As band gap increases, fewer electrons can be excited into the conduction band (make sense?) – material has lower conductivity! Whether a material is an insulator or semiconductor depends on the width of the band gap Another point: increasing temperature usually causes conductivity to increase (why?) Chapter 12 – Electrical Properties • Electron mobility – Electric field applied results in force on free electrons which experience an acceleration in a direction opposite to that of the field – Theoretically there is no interaction between free electrons and a perfect crystal lattice – In practice this is not true – one observes that the current reaches a constant value (immediately) after it is applied – “Frictional” forces – free electrons scattered by imperfections, defects, impurities, etc. • Causes a loss in kinetic energy and change in direction METALS: RESISTIVITY VS T, IMPURITIES • Imperfections increase resistivity --grain boundaries --dislocations --impurity atoms --vacancies These act to scatter electrons so that they take a less direct path. • Resistivity increases with: --temperature --wt% impurity --%CW (cold work, See section on Strain hardening, Ch. 7) Adapted from Fig. 18.8, Callister 6e. (Fig. 18.8 adapted from J.O. Linde, Ann. Physik 5, p. 219 (1932); and C.A. Wert and R.M. Thomson, Physics of Solids, 2nd ed., McGraw-Hill Book Company, New York, 1970.) r r thermal rimpurities r def 8 EX: ESTIMATING CONDUCTIVITY • Question: --Estimate the electrical conductivity of a Cu-Ni alloy that has a yield strength of 125MPa. Adapted from Fig. 7.14(b), Callister 6e. r 30x10 8 Ohm m Adapted from Fig. 18.9, Callister 6e. 1 3.3x10 6 (Ohm m) 1 r 9 Chapter 12 – Electrical Properties • Electron mobility – The scattering phenomenon is manifested as a resistance to the passage of an electric current – Several parameters are used to describe this including the drift velocity and mobility – Drift velocity represents the average electron velocity in the direction of the force imposed by the applied field d e E e – mobility, E – Electric field e [=] m2/V-s • The conductivity of most materials can be expressed as n e e n - # of free e-’s per unit volume Chapter 12 – Electrical Properties • Read 12.8, 12.9! • Semiconductivity – While not as high as the conductivities of metals, semiconductors have unique electrical properties – Why is this? Their electrical properties are extremely sensitive to the presence of minute concentrations of impurities – Intrinsic semiconductors – the electrical behavior is based on the electronic structure inherent in the pure material – Extrinsic semiconductors – the electrical behavior is dictated by impurity atoms Chapter 12 – Electrical Properties • Intrinsic semiconduction – Band gap structure shown below • Two elemental semiconductors are Silicon and Germanium (band gap energies of 1.1 and 0.7 eV respectively) – There are many compounds that exhibit semiconducting properties (GaAs, InSb, CdS, ZnTe) Chapter 12 – Electrical Properties • Intrinsic semiconduction – Hole – for every electron excited into the conduction band there is left behind a missing electron in one of the covalent bonds, or in the band scheme, a vacant electron state in the valence band – Electrons and hole move in opposite directions in the applied field Chapter 12 – Electrical Properties • Intrinsic semiconduction – Two types of charge carrier – the expression for electrical conduction needs to account for this n e e p e h ni e e h Include term for holes – note n = p = ni ni is the intrinsic carrier concentration Chapter 12 – Electrical Properties • Extrinsic semiconduction – Here the key is the ability to control very precisely the impurity content (e.g. 1 atom in 1012!) – Virtually all commercial semiconductors are extrinsic – Two types: n-Type and p-Type • n-Type extrinsic semiconduction – Incorporate impurity atom with more valence electrons than the matrix atoms (P into Si) – The extra non-bonding electron can be readily removed from the impurity atom (interaction w/P ~ 0.01 eV) Chapter 12 – Electrical Properties • n-Type extrinsic semiconduction – From a band gap view – This extra electron (donor impurity) is located within the band gap just below the conduction band (see below) – Since the impurity atom donates an electron to the conduction band this type of impurity is referred to as a donor – Note that here no hole is created within the valence band (why?) At RT sufficient thermal energy to excite large numbers of electrons from donor states Number of electrons in the conduction band far exceeds the number of holes in the valence band n-type extrinsic semiconductor Chapter 12 – Electrical Properties • n-Type extrinsic semiconduction – Few more points • Since the number of electrons in the conduction band far exceed the number of holes in the valence band n e e – The electrons are called the majority carriers, the holes are the minor charge carriers – The Fermi level is shift upward in the band gap, closer to the donor states (exact position depends on T and concentration (sections 12.12, 12.13)) PURE SEMICONDUCTORS: CONDUCTIVITY VS T • Data for Pure Silicon: -- increases with T --opposite to metals undoped e Egap / kT electrons can cross gap at higher T Adapted from Fig. 19.15, Callister 5e. (Fig. 19.15 adapted from G.L. Pearson and J. Bardeen, Phys. Rev. 75, p. 865, 1949.) material Si Ge GaP CdS band gap (eV) 1.11 0.67 2.25 2.40 Selected values from Table 18.2, Callister 6e. 10 CONDUCTION IN TERMS OF ELECTRON AND HOLE MIGRATION • Concept of electrons and holes: • Electrical Conductivity given by: # holes/m3 ne e p e h # electrons/m 3 Adapted from Fig. 18.10, Callister 6e. hole mobility electron mobility 11 INTRINSIC VS EXTRINSIC CONDUCTION • Intrinsic: # electrons = # holes (n = p) --case for pure Si • Extrinsic: --n ≠ p --occurs when impurities are added with a different # valence electrons than the host (e.g., Si atoms) • N-type Extrinsic: (n >> p) • P-type Extrinsic: (p >> n) Phosphorus atom Boron atom hole 4+ 4+ 4+ 4+ n e e 4+ 5+ 4+ 4+ 4+ 4+ 4+ 4+ no applied electric field conduction electron valence electron Si atom 4+ 4+ 4+ 4+ 4+ 3+ 4+ 4+ p e h 4+ 4+ 4+ 4+ no applied electric field 12 Chapter 12 – Electrical Properties • p-Type extrinsic semiconduction – Now the impurity is a trivalent atom (boron or gallium are the typical culprits) – Now one of the covalent bonds is deficient one electron • Hole weakly bond to the atom – Hole can migrate by the transfer of an electron from an adjacent bond – More pictures … Chapter 12 – Electrical Properties • p-Type extrinsic semiconduction – From a band gap picture – Each impurity atom introduces an energy level within the band gap, above yet close to the top of the valence band (often referred to as the impurity electron state) – Hole subsequently created in the valence band – however only one carrier is produced; a hole in the valence band – No free electrons are created Chapter 12 – Electrical Properties • p-Type extrinsic semiconduction – This type of impurity is called an acceptor as it accepts an electron from the valence band and generates a hole – Energy level within the band gap is called an acceptor state – Holes are present in much higher concentrations than free electrons (p >> n) – Material is referred to as p-type because positively charged particles (holes) are primarily responsible for electrical conduction – Holes – majority carriers, electrons – minority carriers p e h Chapter 12 – Electrical Properties • p-Type extrinsic semiconduction • Fermi level is positioned within the band gap and near the acceptor level • Both n- and p- type extrinsic semiconductors are produced from materials initially of extremely high purity (~ 10-7 at%) • Key – very high degree of control over the addition of impurity atoms (doping) Chapter 12 – Electrical Properties • Temperature dependence of Carrier Concentration – Intrinsic semiconductors • Concentration goes up as temperature goes up – why is that? – Extrinsic semiconductors • Things are more complicated here! • Plot below: Si doped w/1021 m-3 P atoms • 3 regions – Lowest T – freeze out region » Charged carriers are “frozen” to dopant atoms – Intermediate region (Extrinsic) » n-type: concentration of carriers is constant » Explain why! – High temperature (intrinsic) » Explain this to me! Chapter 12 – Electrical Properties • Factors that affect carrier mobility – Conductivity of a material is dependent not only on the electron and/or hole concentration, but also their mobility – Dopant content (extrinsic semiconductors) • At low dopant contents (~ 1021 m-3), mobilities are at their maximum • Higher contents, mobility decreases • Note e > h • Carrier mobility • Temperature effects – Mobility generally goes down with temperature (thermal scattering) – Mobility goes down with dopant concentration Chapter 12 – Electrical Properties • Example 12.1 • For intrinsic gallium arsenide (GaAs), the room-temperature electrical conductivity is 10-6 (-m)-1; the electron and hole mobilities are 0.85 and 0.04 m2/V-s. What is ni? ni e h e need ni ni e h e 10 6 m ni 1.6 10 19 C 0.89m 2 / V s 1 ni 7.0 1012 m 3 Chapter 12 – Electrical Properties • Example 12.2 • What is the conductivity of pure (intrinsic) silicon at 150 C How are going to solve this? What do you need? ni e h e How do you get these quantities? Get ni from carrier concentration vs. T (Fig 12.15) ni 4 1019 m 3 Now what ? e 0.06m 2 / V s h 0.022m 2 / V s 4 1019 m 3 1.6 10 19 C 0.082m 2 / V s 0.52 m 1 DOPED SEMICON: CONDUCTIVITY VS T • Data for Doped Silicon: -- increases doping --reason: imperfection sites lower the activation energy to produce mobile electrons. • Comparison: intrinsic vs extrinsic conduction... --extrinsic doping level: 1021/m3 of a n-type donor impurity (such as P). --for T < 100K: "freeze-out" thermal energy insufficient to excite electrons. --for 150K < T < 450K: "extrinsic" --for T >> 450K: "intrinsic" Adapted from Fig. 18.16, Callister 6e. (Fig. 18.16 from S.M. Sze, Semiconductor Devices, Physics, and Technology, Bell Adapted from Fig. 19.15, Callister 5e. (Fig. 19.15 adapted from G.L. Pearson and J. Bardeen, Phys. Rev. 75, p. 865, 1949.) Telephone Laboratories, Inc., 1985.) 13 Chapter 12 – Electrical Properties • Hall Effect – How do you determine the majority charge carrier type, concentration, and mobility? – Can’t get this from conductivity measurements! – Perform Hall effect experiment (see below) • Apply electrical field to sample (left) so that electrons/hole move along x • Apply magnetic field (Bz) along z • This magnetic field induces a voltage perpendicular both to the magnetic field and the current along x • The magnetic field results in a force on the holes/electrons such that they are deflected along y • Gives rise to the Hall voltage (VH) Chapter 12 – Electrical Properties • The magnetic field results in a force on the holes/electrons such that they are deflected along y • Gives rise to the Hall voltage (VH) RH I x Bz VH d • RH is the Hall coefficient, which is constant for a given material • For metals, conduction is by electrons so 1 RH ne e ne and e RH Determining the majority carrier, mobility for semiconductors is more complicated Chapter 12 – Electrical Properties • Semiconductor devices – OK, time for some engineering! – Point: electrical properties of semiconductors allow them to be used to perform electronic functions – diodes, transistors – Advantages: small size, low power consumption, no warmup time – In short, these are the workhorse of the semiconductor industry and why your phone is so small! Chapter 12 – Electrical Properties • p-n Rectifying Junction – Rectifier/diode – permits current to flow in one direction only. A rectifier can transform alternating current to direct current – Construct p-n rectifying junction from a single piece of semiconductor doped such that one side is n-type and the other is p-type • Why can’t you join pieces of n- and p- type materials? No voltage At the junction electrons and holes recombine The p-n rectifying junction • The p-n junction possesses some interesting properties which have useful applications in modern electronics. • P-doped semiconductor is relatively conductive. The same is true of N-doped semiconductor, but the junction between them is a nonconductor. • This nonconducting layer, called the depletion zone, occurs because the electrical charge carriers in doped ntype and p-type silicon (electrons and holes, respectively) attract and eliminate each other in a process called recombination. • By manipulating this nonconductive layer, p-n junctions are commonly used as diodes: electrical switches that allow a flow of electricity in one direction but not in the other (opposite) direction. • This property is explained in terms of the forward-bias and reverse-bias effects, where the term bias refers to an application of electric voltage to the p-n junction. P-n junction: forward bias With this set-up, the ‘holes' in the P-type region and the electrons in the N-type region are pushed towards the junction. This reduces the width of the depletion zo The positive charge applied to the P-type block repels the holes, while the negative charge applied to the N-type block repels the electrons. As electrons and holes are pushed towards the junction, the distance between them decreases. This lowers the barrier in potential. With increasing bias voltage, eventually the nonconducting depletion zone becomes so thin that the charge carriers can tunnel across the barrier, and the electrical resistance falls to a low value. The electrons which pass the junction barrier enter the P-type region and a curren flows. Chapter 12 – Electrical Properties • p-n Rectifying Junction – Thus for a forward bias large numbers of charge carriers move across the semiconductor to the junction • Reverse bias – now switch potential; holes and electrons move away from the junction. Current-Voltage behavior in junctions At high reverse bias voltages (~ 100’s V), large numbers of charge carriers are generated. Gives rise to an abrupt increase in current known as breakdown Forward bias – appreciable current, low resistivity Reverse bias – highly insulative (low current, high resistance) Chapter 12 – Electrical Properties • p-n Rectifying Junction – Great!? What does this have to do with electronics – By switching the bias you control current flow through the junction – Why? Current in the reverse bias mode IR is very small compared to that in forward bias mode IF IR << IF; this is called rectification P-N RECTIFYING JUNCTION • Allows flow of electrons in one direction only (e.g., useful to convert alternating current to direct current. • Processing: diffuse P into one side of a B-doped crystal. • Results: --No applied potential: no net current flow. --Forward bias: carrier flow through p-type and n-type regions; depletion zone is reduced; current flows. --Reverse bias: carrier flow away from p-n junction; carrier conc. greatly reduced at junction; little current flow. 14 Chapter 12 – Electrical Properties • Transistors – Perform two primary functions • Amplify electrical signals • Serve as switching devices for processing/storing information – Talk about two major types here • Junction (bimodal) transistor • Metal oxide semiconductor field-effect transistor (MOSFET) Chapter 12 – Electrical Properties • Transistors – Junction transistors • Basic idea: two p-n junctions arranged back to back (either n-p-n or p-n-p) – talk about p-n-p here • Place thin n-type base region between the two p-type regions; one is called the emitter and the other is called the collector • Note biasing of the circuitry • Transistors – Junction transistors – how they work • Given forward bias, large numbers of holes enter the base region. While some recombine with electrons, if the material is properly designed, most are swept through the base without recombining and into junction two • Since there is a reverse bias on the output side (into the collector), holes are effectively driven away from junction 2 • Small increase in input voltage within emitter-base circuit leads to large increase in current across junction 2 (collector current), leads to large increase in voltage across load resistor Chapter 12 – Electrical Properties • Transistors – Junction transistors – how they work • Aside: can also make n-p-n transistors: main difference – electrons, not holes, are injected across the base and into the collector • Metal oxide semiconductor field effect transistor (MOSFET) – Two small islands of a p-type semiconductor within a substrate of n-type silicon. Islands joined by a narrow p-type channel – Have metal connects to islands; form an insulating layer on the surface by oxidizing silicon • Key point: conductivity of the channel is varied by the presence of an electrical field on the gate • (MOSFET) • Physics: conductivity of the channel is varied by the presence of an electrical field on the gate – Impose positive field on gate – drive charge carriers (holes) out of the channel, reducing conductivity – Small alteration of field at the gate produces a relatively large variation in current between the source and drain – Key difference w/junction transistor – gate current is exceedingly small as compared to the junction transistor – Majority carrier dominates MOSFET behavior, minority carriers play a role with junction transistors Manipulate V at gate to vary current betwee source and drain Chapter 12 – Electrical Properties • Semiconductors in computers – Transistors amplify electrical signals – They can also be used as switching devices • Used for arithmetic, logic and storing functions – Numbers in computers are represented in binary code (0 or 1) – Transistors can operate as a “two state” switch • On or off (conducting/non-conducting) – Please read about microelectronic circuitry (p 507) Chapter 12 – Electrical Properties • Electrical conduction in ionic ceramics & polymers – Short story – most are not good conductors (i.e. they are insulators) – High T – conductivity does go up Read 12.16, 12.17 Chapter 12 – Electrical Properties • Dielectric behavior – A dielectric material is one that is electrically insulating (nonmetallic) and exhibits or can be made to exhibit an electric dipole structure • Huh? It can separate positively and negatively charged entities on a molecular/atomic scale Remember this from physics? • These are used as capacitors Chapter 12 – Electrical Properties • Dielectric behavior – Apply a voltage across a capacitor – one plate is negatively charged, the other is positively charged. The capacitance relates to the amount of charge stored on either plate C Q , C[]C / V or Farad F V – If I have a parallel plate capacitor with vacuum between the plates the capacitance can be calculated as C o A , A plate area, l distance between plates l o Permittivi ty of vacuum (8.85 10 12 F / m) Chapter 12 – Electrical Properties • Dielectric behavior – Now, put a dielectric material between the plates A C l – is permittivity of the material (greater than o). The relative permittivity (dielectric constant) is given by r – Note this quantity is always greater than one, and is the key o property for a capacitor Chapter 12 – Electrical Properties • Field vectors and polarization – Way to describe the phenomena of capacitance – For every electric dipole there is a separation of charge – the electric dipole moment (p) associated with each dipole is p qd – The dipole moment is actually a vector; in the presence of an electric field a force is exerted on the dipole to orient it with the applied field; this is called polarization – For a dielectric material in a capacitor the surface charge density is given as D E – This is also sometimes called the dielectric displacement Chapter 12 – Electrical Properties • Field vectors and polarization – Can explain increase in capacitance using a model of polarization within a dielectric material: consider the figures below In the case above (vacuum) the charge is on the plates In the case above the entire solid (dielectric) becomes polarized. Net accumulation of charge in the dielectric near the plate surface. No net charge in the middle of the material, but the charge accumulation at the edges is facilitated by polarization Chapter 12 – Electrical Properties • Field vectors and polarization – Due to polarization of the dielectric there is accumulation of negative charge (-Q’) near the surface of the positively charged plate – The induced charge from the dielectric can be thought of as nullifying some of the charge that originally existed on the plate when in vacuum – In the presence of the dielectric the surface charge density is now given by E D oE P P o r 1E May be thought of as a polarization E field with the dielectric that results from alignment of the dipoles Chapter 12 – Electrical Properties • Types of polarization – Three sources of polarization: electronic, ionic, orientation – Electronic: can be induced in all atoms. Due to displacement of the center of the electron cloud from the nucleus by the electric field – Ionic: Found in ionic materials. Electric fields displaces cations and anions in opposite directions – Orientation: Only found in substances with a permanent dipole moment • Dipoles align with the field • Frequency dependence of the dielectric constant – This matters because in many cases the current is alternating, or the applied voltage changes direction with time – As voltage “switches” the dipoles attempt to reorient with the field – This reorientation takes some finite amount of time – the relaxation frequency is the reciprocal of the minimum reorientation time What happens if the frequency of the applied electric field exceeds the relaxation frequency of the dielectric material? • Frequency dependence of the dielectric constant – What happens if the frequency of the applied electric field exceeds the relaxation frequency of the dielectric material? • The dipoles cannot keep shifting their orientation – that dipole will not contribute to the dielectric constant • See abrupt changes in r as different mechanisms are “turned off” • Absorption of energy by a dielectric material subjected to an alternating field is called the “dielectric loss”. A low loss is desired – why? Chapter 12 – Electrical Properties • Dielectric strength – What can happen to a material if you put a large voltage/electric field on it? – Can turn it into a conductor! – This can lead to material degradation/failure • Clearly don’t want this to happen! – The dielectric strength is the electric field needed to induce breakdown of a material SUMMARY • Electrical conductivity and resistivity are: --material parameters. --geometry independent. • Electrical resistance is: --a geometry and material dependent parameter. • Conductors, semiconductors, and insulators... --different in whether there are accessible energy states for conductance electrons. • For metals, conductivity is increased by --reducing deformation --reducing imperfections --decreasing temperature. • For pure semiconductors, conductivity is increased by --increasing temperature --doping (e.g., adding B to Si (p-type) or P to Si (n-type). 15 ANNOUNCEMENTS Reading: Chapter 12 HW 10: Due Monday, April 9th Problems 12.1; 12.3; 12.6; 12.8; 12.11; 12.16; 12.19; 12.27; 12.28; 12.35; 12.40; 12.43 0