* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 16-5 and 16-6 Coulomb`s Law
Survey
Document related concepts
Elementary particle wikipedia , lookup
Anti-gravity wikipedia , lookup
Newton's theorem of revolving orbits wikipedia , lookup
Newton's law of universal gravitation wikipedia , lookup
Electromagnetism wikipedia , lookup
Nuclear force wikipedia , lookup
Fundamental interaction wikipedia , lookup
Newton's laws of motion wikipedia , lookup
Classical central-force problem wikipedia , lookup
Work (physics) wikipedia , lookup
Lorentz force wikipedia , lookup
Transcript
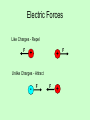
Chapter 16 Electric Charge and Electric Field Objectives: The students will be able to: Apply Coulomb's law to determine the magnitude of the electrical force between point charges separated by a distance r and state whether the force will be one of attraction or repulsion. Two types of charge: Positive Charge: A shortage of electrons. Negative Charge: An excess of electrons. Conservation of charge – The net charge of a closed system remains constant. Nucleus - - n + n + + n + n n + + n - - - - Negative Neutral Atom Atom Positive Atom Number Numberof ofelectrons electrons><=Number Numberof ofprotons protons Number of electrons Number of protons -19 -2e = -3.2 x 10 CC +2e = +3.2 x 10-19 Inquiry Activity Electric Field Hockey phET Electric Forces Like Charges - Repel F + + Unlike Charges - Attract - F F + F 16.5 Coulomb’s Law Experiment shows that the electric force between two charges is proportional to the product of the charges and inversely proportional to the distance between them. Coulomb’s Law – Gives the electric force between two point charges. q1q2 F k 2 r Inverse Square Law k = Coulomb’s Constant = 8.988x109 Nm2/C2 q1 = charge on mass 1 q2 = charge on mass 2 r = the distance between the two charges The electric force is much stronger than the gravitational force. 16.5 Coulomb’s Law Coulomb’s law: (16-1) This equation gives the magnitude of the force. 16.5 Coulomb’s Law The force is along the line connecting the charges, and is attractive if the charges are opposite, and repulsive if they are the same. 16.5 Coulomb’s Law Unit of charge: coulomb, C The proportionality constant in Coulomb’s law is then: Charges produced by rubbing are typically around a microcoulomb: Coulomb's Law • The force between two charges gets stronger as the charges move closer together. • The force also gets stronger if the amount of charge becomes larger. Coulomb's Law • The force between two charges is directed along the line connecting their centers. • Electric forces always occur in pairs according to Newton’s third law, like all forces. Coulomb's Law • The force between charges is directly proportional to the magnitude, or amount, of each charge. • Doubling one charge doubles the force. • Doubling both charges quadruples the force. Coulomb's Law • The force between charges is inversely proportional to the square of the distance between them. • Doubling the distance reduces the force by a factor of 22 = (4), decreasing the force to onefourth its original value (1/4). • This relationship is called an inverse square law because force and distance follow an inverse square relationship. 16.5 Coulomb’s Law Charge on the electron: Electric charge is quantized in units of the electron charge. Unit of charge is a Coulomb (C) Example 1 Two charges are separated by a distance r and have a force F on each other. qq F k F 1 2 2 r q2 q1 F r If r is doubled then F is : ¼ of F If q1 is doubled then F is : 2F If q1 and q2 are doubled and r is halved then F is : 16F Example 2 Two 40 gram masses each with a charge of 3μC are placed 50cm apart. Compare the gravitational force between the two masses to the electric force between the two masses. (Ignore the force of the earth on the two masses) 3μC 40g 3μC 40g 50cm m1m2 Fg G 2 r 6.67 10 11 (.04)(.04) 2 (0.5) 4.27 10 q1q2 FE k 2 r 6 6 ( 3 10 )( 3 10 ) 9 9.0 10 (0.5) 2 13 N 0.324 N The electric force is much greater than the gravitational force Homework Problems page 465 #s 1, 3, 5, 7, 10 Objectives: The students will be able to: • Apply Coulomb's law to determine the magnitude of the electrical force between point charges separated by a distance r and state whether the force will be one of attraction or repulsion. • For practical reasons, the coulomb is defined using current and magnetism giving k = 8.988 x 109 Nm2/C2 • Permittivity of free space e0 = Then 1 4p k = 8.85´10 1 q1q2 F= 2 4pe0 r -12 2 C / Nm 2 16.5 Coulomb’s Law The proportionality constant k can also be written in terms of , the permittivity of free space: (16-2) Coulombs Law Lab Experiment In 1785 Charles Augustin Coulomb reported in the Royal Academy Memoires using a torsion balance two charged mulberry pithballs repelled each other with a force that is inversely proportional to the distance. kq1q 2 F= 2 r q1 r where k=8.99*109 Nm2/C2 in SI unit k ~ 1010 Nm2/C2 + q2 Point charges Spheres same as points + Repulsion + - -- Attraction Repulsion Summer July 06 PHYS632 E&M 24 Superposition of electric forces Net force is the vector sum of forces from each charge F3 q1 q2 q q3 F2 F1 Net force on q: F = F1 + F2 + F3 F Principle of Superposition Three charges In a line • In the previous example we tacitly assumed that the forces between nuclei simply added and did not interfere with each other. That is the force between two nuclei in each penny is the same as if all the others were not there. This idea is correct and is referred to as the Principle of Superposition. • Example of charges in a line x 2 1 – 3 Three charges lie on the x axis: q1=+25 nC at the origin, q2= -12 nC at x =2m, q3=+18 nC at x=3 m. What is the net force on q1? We simply add the two forces keeping track of their directions. Let a positive force be one in the + x direction. æ q2 q3 ö F=-kq1 ç + è (2m)2 (3m)2 ÷ø ( = - 10 10 Nm 2 C2 = 2.5 ´ 10 -7 N ) æ -12 ´ 10 -9 C 18 ´ 10 -9 C ö (25 ´ 10 C) ç + 2 (3m)2 ÷ø è (2m) -9 Force from many charges Q2 F41 Q1 - F21 + F31 Principle of superposition - + Q4 Q3 Force on charge is vector sum of forces from all charges F1 F21 F31 F41 Example 16-3 p.448 Three charges in a line. Three charged particles are arranged in a line, as shown below. Calculate the net electrostatic force on particle 3 (the -4.0μC on the right) due to the other two charges. Example 16-3 p.448 Three charges in a line. Three charged particles are arranged in a line, as shown below. Calculate the net electrostatic force on particle 3 (the -4.0μC on the right) due to the other two charges. 16.5 Coulomb’s Law Coulomb’s law strictly applies only to point charges. Superposition: for multiple point charges, the forces on each charge from every other charge can be calculated and then added as vectors. 16.6 Solving Problems Involving Coulomb’s Law and Vectors The net force on a charge is the vector sum of all the forces acting on it. 16.6 Solving Problems Involving Coulomb’s Law and Vectors Vector addition review: Example 3 Three charged objects are placed as shown. Find the net force on the object with the charge of -4μC. F k - 5μC 45º 20cm 202 202 28cm q1q2 r2 (5 106 )(4 106 ) F1 9 10 4.5N 2 (0.20) 9 (5 106 )(4 106 ) F2 9 10 2.30 N 2 (0.28) 9 F1 45º - 4μC 5μC 20cm F2 F1 and F2 must be added together as vectors. F1 2.3cos45≈1.6 45º F2 2.3sin45≈1.6 F1 = < - 4.5 , 0.0 > + F2 = < 1.6 , - 1.6 > Fnet = < - 2.9 , - 1.6 > - 1.6 - 2.9 29º θ 3.31 Fnet 2.9 2 1.6 2 3.31N 1.6 tan 29 2.9 1 3.31N at 209º Homework Problems on pages 465 and 465 #s 12, 13, 18 Closure Kahoot