* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Jenkin_TB_PBL
Compartmental models in epidemiology wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Public health genomics wikipedia , lookup
Focal infection theory wikipedia , lookup
Epidemiology of HIV/AIDS wikipedia , lookup
Diseases of poverty wikipedia , lookup
Infection control wikipedia , lookup
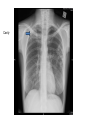
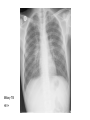
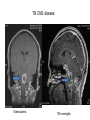
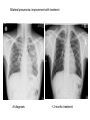
Paper PBL 15 – Extreme Lethargy Group 4 MMC Kate, James, Lee, Quaderi, Jeeves, Satwik, Helen, Shravya, Jo, Nikhil Case History • Mr Josh Felix • 25 years old, roadie for a “grunge band” • Grew up in Wagga Wagga, moved to Glen Waverly 5 years ago Presenting Complaint: • 6 week history of increasing lethargy, productive cough, weight loss he assumed it was exacerbation of asthma • Assuming it was asthma, he attended 24 hour medical clinic for repeat prescription of asthma medications given salmeterol, fluticasone inhalers, prednisolone 5mg and amoxycillin 500mg for his cough. • Returned 5 days later due to worsening symptoms. New doctor on duty takes thorough history and examination to find… Josh’s History • Productive cough – sputum thick, light brown, coughing one “table spoon” each morning • No haemoptysis • Mild dsypnoea on exertion • No chest pain • Fever; chills & muscle aches followed by profuse sweating • Asthma since age 5; 3-4 attacks each year; uses inhalers intermittently • No other medications • Smokes 25 cigarettes per day, done so for 7 years • Non-IVDU; Alcohol: 4 drinks per day + 2-3 binges per month Further Relevant History • FHx – Josh’s mother was treated for “spitting blood” 18 years ago. Brother has severe asthma. • Contact Hx – members of band have a “cold” • Sexual Hx – many different female partners, often unprotected. Sex with a man on once. • Travel Hx – Never travelled overseas. Recently spent 2 months in Darwin. • Animal contact – none of relevance • Immunisations – can’t be recalled • Dietary Hx – erratic diet, mainly junk food, no fresh fruit or vegetables Physical Examination • • • • • • • Gaunt, white male; not acutely ill. Pulse 98/min BP 130/74 mmHg RR 16/min Oral temp 37.6°C Weight 58kg Hyperexpanded chest, soft rhonchi bilaterally, no other focal resp signs • CVS normal, no hepatomegaly • Additional notes of tattoos, multiple piercings, cigarette pack in t-shirt sleeve and no BCG scar Initial Investigation Results • FBE: Hb 109g/L, WBC 14.5x10^9/L, platelets 140x10^9/L • HIV serology: negative • LFT’s: – – – – – bilirubin 19 (N<17) ALP 110 (N<120) ALT 240 (N<56) GGT 150 (N<75) Albumin 26 g/L (N35-45) • CXR: hyperexpanded lung fields, right apex opacity with 2x2cm cavity, no cardiomegaly, hilar regions normal • Sputum Gram stain: WBC +++, mixed Pos and Neg organisms • Sputum Culture: normal oral flora • Special Cultures: – Burkholderia pseudomallei: pending – AFB stain: positive ++ (first specimen) – AFB culture: in progress Summary of Findings • • • • • • • • • • • • • • • Country of birth: Australia Productive cough – sputum thick, light brown, coughing one “table spoon” each morning Mild dsypnoea on exertion Fever; chills & muscle aches followed by profuse sweating Symptoms progressively worse over 6 weeks with weight loss FHx – Josh’s mother was treated for “spitting blood” 18 years ago. Sexual Hx – many different female partners, often unprotected. Sex with a man on once. Travel Hx – nil overseas, 2 months in Darwin. Immunisations – can’t be recalled, no HBG scar 7 pack years smoking, high alcohol intake, poor nutrition Gaunt, white male; not acutely ill, weight 58kg Hyperexpanded chest, soft rhonchi bilaterally, no other focal resp signs LFT’s: intrahepatic pattern with GGT CXR: hyperexpanded lung fields, right apex opacity with 2x2cm cavity, no cardiomegaly, hilar regions normal Sputum AFB stain: positive ++ Differential Diagnosis •Tuberculosis •Pneumonia/Atypical Pneumonia •Asthma exacerbation •COPD •Bronchiectasis •Lung carcinoma •HIV •Lung abscess Risk Factors for disease • Lowered immunity – HIV, diabetes, ESRD, certain neoplasms (lung, lymphoma) – Corticosteroids, transplant drugs – Age • Silicosis • Ethnicity – Prolonged residence in TB endemic country – Sub-saharan Africa, SE Asia, Subcontinent Risk Factors for exposure • Known contact with someone with TB • Prolonged Travel to high-risk areas • Health-Care work Epidemiology – World Wide • • • • No. 1 infectious disease problem > 2 billion people are infected with TB bacilli 9.4 million new cases (2008) 1.8 million people died (2008) – 500,000 had HIV Epidemiology – World Wide • South-east Asia – Highest incidence and deaths – Approx 3.2 million new cases (2008) • Sub-Saharan Africa – Highest incidence and mortality rate – Approx 2.8 million new cases (2008) Global TB incidence •2 billion infected •9.4 million cases 2008 •1.3 million deaths • 95% cases occur in developing world WHO 2009 Global TB incidence rates Global incidence rate 139/100000/yr WHO 2009 Epidemiology - Australia Epidemiology - Australia Investigations I(x) Active TB Routine; FBE •↑ WCC (Infection) •↓ Hb (Anaemic of chronic disease) U&E’s •(baseline) LFT’s •(baseline) ESR/CRP •(inflammation/infection) I(x) Active PTB Diagnostic Chest X-Ray • Abnormal CXR often found with no symptoms but reverse extremely rare • PTB is unlikely in absence of radiographic abnormalities • Exception is miliary TB or non-respiratory TB Findings • Patchy or nodular shadows in the upper zones • Loss of volume and fibrosis (with or without cavitation) • Calcification may be present Similar CXR findings • Histoplasmosis, fungal infections (cryptococcosis, coccidiomycosis, blastomycosis, aspergillosis), bronchial carcinoma, cavitating pulmonary Infarcts EVERY EFFORT MUST BE MADE TO OBTAIN MICROBIOLOGICAL EVIDENCE Cavity Miliary TB HIV+ I(x) Active TB Culture Clinical Samples • sputum, pleura & pleural fluid, urine, pus, ascites, bone marrow, CSF • Induce if non-productive (bronchoscopy & lavage) • Prolonged culture – 12wks AFB – acid fast bacilli • Ziehl-Neelsen stain • Acid fast bacilli are stained bright red and stand out against a blue background • Resistant to de-colouring when washed with acid I(x) Active TB Other • Imaging for non-respiratory TB (CT, XR etc) • PCR – rapid identification of sensitivity/resistance (rifampicin) • Biopsies – pleura, lymph nodes, solid lesions etc I(x) Latent TB • When infected with M Tuberculosis, but do not have active tuberculosis disease. • Patients are not infectious. • TB infections in Australia are predominantly due to reactivation of latent infection in people who were previously infected in their countries of birth or during their childhood when TB was more common in Australia. • Simply put, the immune system ‘walls off’ the TB bacilli (in a granulomatous lesion), which can lie dormant for years. It is kept in this state by the cell-mediated immune system. • Main Risk: around 10% of these people will develop active TB during some point in their lives – the greatest risk being within the first 2 years of being infected. • Usually when their immune system is weakened. Investigations – Mantoux Test (Tuberculin Skin test= TST) • Readily available test for identifying latent M. tuberculosis infection. • Works via a hypersensitivity reaction by the cell-mediated immune system to purified proteins from M. Tuberculosis (called Tuberculin). • Tuberculin is injected intradermally in the forearm and the resulting area of induration (not erythema) is measured 48-72 hours later. • Positive result is based on the size of the induration, considering the risk-status and prevalence of TB in certain patients. • Previous vaccination with BCG affects the way results are interpreted – may give false positives. • Mantoux test should be done to identify people with an increased risk of TB, who would benefit from treating the latent infection. – People with HIV, recent contacts of a person known to have clinically active TB, health care workers at increased risk, etc. Tuberculin= Purified Protein derivative (PPD)- intredermally 48-72 h Transverse diameter of induration in mm Criteria for TST positivity Induration Result Group > 5 mm Positive HIV-infected Immunosuppressed (TNFa, Tx, Close contacts of infectious TB Old TB on CXR > 10 mm Positive Medical risk factors (CRF, CA etc.) Foreign born endemic TB area HCW Nursing home, prisoners > 15 mm Positive All other persons BCG vaccinated Investigations – QuantiFERON-TB Assay • A recently produced blood test that is able to measure quantitatively the production of cytokine Interferon-γ by lymphocytes sensitised to mycobacterial proteins using an ELISA technique. • Advantages: – Involves only 1 visit for a blood sample. – No injection technique/subjective interpretation problems – Does not boost responses measured by subsequent tests, which can happen with tuberculin skin tests – Is not affected by prior BCG vaccination. Pathophysiology of TB The Pathogens • TB is mainly caused by Mycobacterium tuberculosis. • It can occasionally be caused by M. bovis or M. africanum. • M. tuberculosis divides every 15-20 hours. • It is has a thick cell wall rich in lipids which prevents it taking up most stains and helps it resist digestion in macrophages. • It is an aerobe & an acid fast bacillus. Infection & Dormancy • M. tuberculosis is spread in aerosols released by coughing/sneezing. It needs to be inhaled for infection to occur. • Once inhaled, the bacteria reach the alveoli and are phagocytosed by the alveolar macrophages. Their lipid coating and ability to inhibit phagosome-lysosome fusion enables them to avoid digestion. • This primary infection site is called a Ghon focus and is usually in the lower part of the upper lobe or the upper part of the lower lobe. • The bacteria soon reach the lymph nodes at the hilum of the lung. The ghon focus and the infected node constitute a Ghon complex. These are visible on X ray. Infection & Dormancy ctd. • The cell mediated immune reaction causes the formation of granulomas. • These are composed of numerous leukocytes surrounding a core of infected macrophages. • Most of the bacteria are destroyed but some enter a dormant state and survive by slowing down their metabolism. • Cells in the centre of the granulomas undergo necrosis. The resulting dead matter looks pale and cheesy and is called caseous necrosis. • Some granulomas undergo calcification and can be seen on X-rays after the disease ceases to be active. Reactivation • The primary infection may not be self limiting if the host is very young/old or immunocompromised. • When the immune system is compromised in someone with latent TB (eg- HIV, diabetes, steroids) the M. tuberculosis can reactivate and cause secondary TB. • Unlike the primary infection this is not self limiting. • The bacteria can spread to many parts of the body and cause serious illness- eg: GIT, brain, liver Clinical Manifestations Clinical Manifestations of TB • Pulmonary disease – Primary disease • Occurs soon after the initial infection in areas of high TB transmission, often in children. • Generally spreads to the upper zones of the lung • The lesion which is formed after infection is usually peripheral and is often accompanied by hilar or paratracheal lymphadenopathy. • The initial lesion heals spontaneously in the majority of cases and may later be seen as a small calcified nodule (Ghon lesion) • However in children and immunocompromised people, the lesion can increase in size and result in either a pleural effusion due to infiltration of bacteria into the pleural space, or the primary site may rapidly enlarge causing central necrosis and cavitation. • Enlarged lymph nodes may compress bronchi, creating obstruction and hence segmental or lobar collapse. • This presents generally with fever, malaise, cough, weight loss and haemoptysis. • There may also be a small pleural effusion or erythema nodosum due to hypersensitivity reaction to the infective proves. Clinical Manifestations of TB • Pulmonary disease – Post-primary • Also known as reactivation TB, this results from endogenous reactivation of latent TB. • This also favours the upper zones. • Typically there is a gradual onset of symptoms over weeks to months. • Presents with lethargy, malaise, anorexia and loss of weight with a fever and couch. • Sputum may be mucoid, purulent or blood-stained. A pleural effusion or pneumonia may be the presenting feature. • On examination, finger clubbing may be present in advanced disease. Often there are no physical signs in the chest though occasionally persistent crackles can be heard. • Signs of pleural effusion, pneumonia and fibrosis may be seen. Clinical Manifestations of TB • Extrapulmonary disease – Miliary or Disseminated Tuberculosis • Due to haematogenous spread of bacteria and can be due to either primary infection or reactivation. • Nonspecific signs such as fever, night sweats, anorexia, weakness and weight loss are the presenting symptoms. • Eventually liver and spleen enlarge and tubercle lesions will appear – Tuberculous meningitis • Seen most often in children or immunocompromised adults. • Results from haematogenous spread of pulmonary disease. • May present with headache and slight mental changes, weeks of lowgrade fever, anorexia, malaise, anorexia and irritability. • May evolve acutely with severe headache, confusion, lethargy, altered sensation and neck rigidity. • Diagnosed via LP and if unrecognised it can be fatal. Clinical Manifestations of TB • Extrapulmonary disease – Cardiac • Pericarditis and pericardial effusions • This can lead to constrictive pericarditis due to fibrosis and calcification an can be fatal. – Eyes • Choroiditis – Genitourinary • Pyuria and haematuria, flank pain, frequency, dysuria, nocturia – GIT • Peritoneal TB causing abdominal pain and GI upset (AFB in ascites). – Skeletal • Vertebral collapse, septic arthritis and osteomyelitis – Skin • Jelly-like nodular rash (lupus vulgaris) and possible erythema nodosum due to hypersensitivity reaction to infection TB CNS disease Tuberculoma TB meningitis Treatment Treatment • Bed rest doesn’t affect outcome • Hospitalisation: – Ill, smear positive, highly infectious patients – Esp in multi-drug resistant TB • Continuous self-admin of drugs for 6 months vital for successful Rx – Lack of compliance 5% pts unresponsive to Rx – Resistance to anti-TB drugs increasing • Isoniazid resistance 10% • Multidrug resistance 1% • Before treatment: – Test FBC, liver, and renal function • Need to alter dosages in pts with liver/renal failure – Test colour vision & acuity • Ethambutanol can cause (reversible) ocular toxicity Treatment • 6 months – Rifampicin 600-900 mg, daily – Isoniazid 300 mg daily – Pyrazinamide 2.5g, 3/week • First 2 months – Ethambutanol 30 mg/kg 3/week • First 2 months • Longer regimen: – For bone TB (9 months), tuberculosis meningitis (1yr) • NEVER use monotherapy – Except when using Isoniazid for latent TB Rx • DOTS: Directly Observed Therapy (short-course) – WHO incentive, to improve detection and compliance – DOT plan: treating physician/TB nurse – Bi-weekly, thrice-weekly treatment instead of daily Treatment of Tuberculosis Standard Short Course 2 months daily (H) Isoniazid (R) Rifampicin (Z) Pyrazinamide (E) Ethambutol ‘Initial phase’ 4 months (H) Isoniazid (R) Rifampicin Daily, or 3-5x/wk ‘Continuation phase’ Bilateral pneumonia- improvement with treatment At diagnosis + 2 months treatment Side Effects • Rifampicin: – – – – – Hepatitis Small rise in AST acceptable Stop if bilirubin rises Orange discolouration of urine & tears Inactivation of the Pill • Isoniazid – – – – Hepatitis Neuropathy Pyridoxine deficit Agranulocytosis • Ethambutanol – Optic neuritis (colour vision fist to deteriorate) – Pyrazinamide: Hepatitis – Athralgia (CI: gout, prophyria) Resistance • Seen in non-compliant pts • MDR (multi-drug resistance) – High mortality (esp in HIV pts) • Use at least 3 drugs to which organism is sensitive • Follow-up – Patients should be seen regularly for duration of chemotherapy – Once more after 3 months to check for relapse • Chemoprophylaxis: – Pts with x-ray changes compatible with TB, but about to undergo immunosuppresive long-term Rx (ie dialysis) – Isoniazid 300-450 mg/day Drug Resistance Mono-resistant TB – resistant to only one drug Poly-resistant TB – resistant to more than one drug but not the combination of isoniazid and rifampicin. Multidrug-resistant TB (MDR-TB) • TB caused by bacteria resistant to at least isoniazid and rifampicin. Extensively drug-resistant TB (XDR-TB) • TB caused by bacteria resistant to isoniazid and rifampicin (i.e. MDR-TB) plus any fluoroquinolone and any second-line anti-TB injectable drugs (amikacin, kanamycin or capreomycin) There is an estimated 150 000 deaths per year from MDR-TB alone. Proportion MDRTB in new cases 1994-2009 • Result from either primary infection with resistant bacteria or may develop secondarily in the course of treatment due to inadequate treatment regimens or poor compliance. • Risk factors include – 1. Previous treatment for TB especially if prolonged 2. Contact with a patient known to have drug resistant TB or live in an area with high drug-resistant TB prevalence 3. Immunocompromised (HIV in particular) 4. Poor compliance 5. Culture +ve after 2 months treatment • Can take up to 2 years to treat with drugs less potent, more toxic and more expensive. Higher mortality rate. • Treatment is based on sensitivity testing with at least 3 drugs and an initial bactericidal injectable agent. • Fluoroquinolone should be used where possible. Resistance Alternative INH, RIF LEVO, PZA, EMB, AMK INH, RIF, EMB LEVO, PZA, AMK, CS +/- PAS/ETH INH, RIF, PZA LEVO, EMB, AMK, CS +/- PAS/ETH INH, RIF, PZA, EMB LEVO, AMK, CS, PAS/ETH, +/- one more drug XDR-TB Linezolid becomes mainstay treatment. Surgery is a limited option if disease localised. Prevention • Bacille Calmette Guerin (BCG) Vaccine • Live attenuated strain of Mycobacterium bovis; 1921 • Efficacy • Clinical trials UK: protective effect of 60 to 80%; • Trials elsewhere have shown variable results; efficacy the closer one gets to the equator – Meta-analysis Colditz et al. (1994) = 50 per cent effective • Most important protective benefits are in minimising the risk of death, meningitis and miliary disease in neonates and young children • WHO recommend given to all children in countries highly endemic for TB Australian Recommendations: 1. Aboriginal neonates in areas of high incidence (e.g. NT, Far North Queensland, N WA & SA) 2. Individuals travelling to or living in areas with prevalence of TB 3. Neonates born to parents with leprosy or a Fx of leprosy; 4. HCWs who may be at high risk of exposure to drug resistant cases • Very safe – Anaphylactoid reactions (rare); Most common is development of a localised abscess at the site of injection, especially if the vaccination is given too deeply – Immuno-compromised risk for disseminated BCG infection • C/I: Positive TST of greater than 5-mm diameter in duration; Immunocompromised (HIV, corticosteroids, chemo, malignancies ); Pregnant (?); PHx TB; Febrile; Pt suffers from a generalised skin disease such as eczema and psoariasis Method • Tuberculin skin test (Manoux) is done first (except infants below 6 months where Hx of TB excluded) • C/I if reactive - risk of severe local inflammation and scarring • Single intradermal injection at the insertion of the deltoid – other sites: risk of keloid formation Infection Control • Isolation = Negative Pressure Rooms – prevent crosscontaminations from room to room – Generates negative pressure to allow air to flow into the isolation room but not escape from the room • Educate patient about transmission cover mouth when coughing etc Prophylaxis (Isoniazid Preventive Treatment=IPT) • Screen close contacts with tuberculin test +CXR – Tuberculin +ve, CXR –ve: nothing further – Pt with HIV, no BCG - isoniazid prophylaxis ↓risk by 40% – Child with +ve tuberculin test treatment – Tuberculin –ve in children/young adults repeat in 6 wks; administer BCG if still –ve / treatment if +ve – Children <1y with family member with TB isoniazid 6/12 + BCG with strain resistant to isoniazid – Overall IPT 70-90% effective HIV TB • Worldwide TB is the most important opportunistic infection in HIV patients – its the commonest killer. • Around 20 million people worldwide are co infected with HIV and TB. • Dual infection of HIV and TB is very low in Australia (sub Saharan Africa > 70%). < 5% of AIDS patients in Australia develop active TB. • 1-7% of the HIV infected people with latent TB, will go on to develop active TB each year – a risk that is 4-25x higher than in non-HIV patients. • TB affects the course of HIV infection: in vitro cytokines released because of MTB enhances HIV replication. • HIV patients newly infected with MTB are more likely to develop symptomatic primary infection. Estimated HIV prevalence in TB cases • Clinical manifestation depends on: – CD4 status (level of immunosuppresion) – Whether the TB is from recently acquired TB or from a reactivation of latent TB. • HIV patients with preserved CD4 counts usually present with pulmonary TB. • Atypical manifestations, extra pulmonary or disseminated TB are more common in: – HIV patients with primary TB – Those with reactivated TB – Impaired immunity ( * CD4 count < 200 per microlitre) Characteristic Late HIV infection * Early HIV infection Pulmonary : extra pulmonary disease 50:50 80:20 Clinical presentation Often resembles primary TB Often resembles post-primary TB Intrathoracic lymphadenopathy Common Rare Lower lobe involvement Common Rare Cavitation Rare Common Tuberculin response Rare Common Sputum smear positivity Less common Common Adverse drug reactions Common Rare Relapse after treatment Common Rare Chest radiograph • Tuberculin skin test or Quantiferon should be part of the routine tests of every newly diagnosed HIV infection – test for latent TB. • Also all newly diagnosed patients with TB should be asked for HIV risk factors, and tested for HIV. • A Mantoux rxn of > 5mm or positive Quantiferon indicates TB infection in people with HIV. • Diagnosis can be tricky particularly in advanced HIV: – Frequently negative sputum smear findings – Atypical radiographic findings – Higher prevalence of extra-pulmonary TB at inaccessible sites – Resemblance to other opportunistic pulmonary infections • Rx of TB in HIV patients is complicated – only managed by expert doctors. • Rifampicin has pharmacokinetic interactions with protease inhibitors (PI) – via hepatic cytochrome p450. • overlapping toxicities between HAART and anti-TB drugs – hepatotoxicity, peripheral neuropathy and GI side effects. • In HIV patients with TB, Rifampicin containing Rx recommended • When to start HAART – Low CD4- 2 weeks after commencing TB therapy – Higher CD4- delay till 2 months or end of TB therapy • With those on HAART: – Rifabutin is used instead of rifampicin if Protease inhibitor used. – Or rifampicin could be used with efavirenz – Isonazid, ethambutol and pyrazinamide are used in standard doses. • Paradoxical treatment rxn – patients who begin HAART and anti-TB drugs at same times can develop fever, lymph gland enlargement or pulmonary infiltration week later – due to heightened immune response to MTB secondary to HAART therapy. Tuberculosis Quiz What do all of these people have in common... ...they’ve all had tuberculosis John Keats – english poet. 2009 movie Bright Star with Abbie Cornish. Singer Cat Stevens (Yusam Islaf) was close to death with TB in 1969. Nicole Kidman’s character Satine, died from TB in Moulin Rouge Singer Tom Jones, of“It’s not unsual” fame. Had TB at each 12 (obviously recovered).