* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 2012 Dyslipidemia Guidelines: Statin Intolerance and Adverse Effects
Survey
Document related concepts
Transcript
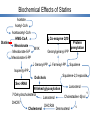
Statin Intolerance and Adverse Effects Canadian Working Group Consensus David Fitchett MD Division of Cardiology St Michael’s Hospital University of Toronto Toronto, Ontario G. B. John Mancini MD Division of Cardiology Department of Medicine University of British Columbia Vancouver, British Columbia Robert Hegele MD Department of Medicine and Robarts Research Institute Schulich School of Medicine London, Ontario Disclosures D Fitchett MD Consulting fees and CME honoraria from Merck, Astra Zeneca, Pfizer, Novartis, Bayer, Sanofi Aventis, Valeant R Hegele MD Consulting fees and CME honoraria from Merck, Astra Zeneca, Pfizer, Amgen, Sunovion, Genzyme, Valeant G. B. J. Mancini MD CME Honoraria from Merck, Astra Zeneca Secondary Review Panel Dominic Ng University of Toronto Milan Gupta McMaster University Jiri Frohlich University of British Columbia Jean Bergeron Laval University Jacques Genest McGill University Janet Pope University Western Ontario Steven Baker McMaster University 54 Year Old Male No prior illness / medications STEMI 5 weeks ago Discharged from hospital on atorvastatin 80mg daily Other medications: ASA, clopidogrel, perindopril, bisoprolol Within a few days, complained of back and lower limb pains and lower limb weakness Also complains of insomnia, and poor memory Biochemistry (on treatment) • CK 200 U/L • ALT 95 U/L How would you manage ? TC 3.5, HDL 1.1, TG 1.4, LDL 1.8 mMol/l, Creatinine 95 μm/l Canadian Journal of Cardiology 2011; 27:635-662 Prospective meta-analysis: 90,056 participants in 14 randomized statin trials For each 1 mmol/L LDL-C lowering 12% reduction in all-cause mortality (P<0.0001) 19% reduction in coronary mortality (P<0.0001) 23% reduction in MI and coronary death (P<0.0001) 24% reduction in revascularizations (P<0.0001) 17% reduction in fatal or non-fatal stroke (P<0.0001) 21% reduction in any major vascular event (P<0.0001) No increase in non-vascular mortality or cancers Adapted from Baigent C et al. Cholesterol Treatment Trialists’ (CTT) Collaborators. Lancet 2005; 366:1267-78 Benefits of More vs Less Intensive Statin Therapy: (5 RCTs, N=39,612) Intensive therapy statin therapy resulted in a further reduction of LDL-C of 0.51mmol/L After 1 year: • 15% reduction in major vascular events • 13% reduction of coronary death or non-fatal MI • 16% reduction in ischaemic stroke CTT Lancet 2010; 376: 1670-81 Effect of Statins on CV Event Rates Related to 1 mmol/l LDL Reduction More statin Less statin A. More statin vs less statin (5 trials: 0.51 mmol/L LDL difference) Any major coronary event 1725 (1.9) Any coronary revascularization 2250 (2.6) Any stroke 572 (0.6) 5 trials: any major vascular event 3837 (4.5) Relative risk per 1 mmol/L (39 mg/dl) reduction in LDL-C (95% CI) 1973 0.74(2.2) (0.65-0.85) 2741 0.66(3.2) (0.60-0.73) 663 0.74(0.7) (0.59-0.92) 4416 0.72(5.3) (0.66-0.78) 0.50 0.75 1.00 1.25 1.50 More statin better Less statin better Statin Control B. Statin vs control (21 trials: 1.07 mmol/L LDL difference) Any major coronary event 3380 (1.3) Any coronary revascularization 3103 (1.2) Any stroke 1730 (0.7) 21 trials: any major vascular event 7136 (2.8) 4539 0.76(1.7) (0.73-0.79) 4066 0.76(1.6) (0.73-0.80) 2017 0.85(0.8) (0.80-0.90) 8934 0.79(3.6) (0.77-0.81) 0.50 0.75 1.00 1.25 1.50 Statin better Control better CCT Trialists. Lancet 2010;376:1670 Absolute Benefit of Statins Event rate (%) Events Prevented by Reducing LDL 1 mmol for 5 yrs 30 25 20 NNT 33 Secondary Prevention 15 10 5 0 Major coronary Coronary revasc. event Outcomes avoided per 1000 30 (24-37) (95% CI) Event rate (%) 37 30 25 20 15 10 5 0 27 (20-34) 125 20 Treatment Control Stroke Major vascular event 8 (4-12) 48 (39-57) 200 40 Stroke Major vascular event 5 (1-8) 25 (19-31) Primary Prevention NNT 55 83 Major coronary Coronary revasc. event Outcomes avoided per 1000 18 (14-23) (95% CI) 12 (9-16) CTT Lancet 2005; 366:1267 Reported Adverse Effects of Statins Muscle-related symptoms Elevated hepato-cellular enzymes Cancer New diabetes Hemorrhagic stroke Fatigue Neuro-psychiatric effects and insomnia Proteinuria / hematuria Erectile dysfunction Alopecia Lack of Risk of Cancer with Statins Meta-analysis of 175,000 subjects in 27 trials 5 years of statin therapy had no effect on the incidence of, or mortality from cancer Incidence of or mortality from cancer not related to • Duration of treatment • Age • Baseline LDL-C level • Sex • Anatomical site of cancer • Risk of vascular event • Type statin CTT Lancet DOI:10.1016/S0140 -6736(12)60367-5 Liver Injury Associated with Statin Use Type of liver injury Frequency Comment Asymptomatic elevations in aminotransferases 0.1%-3.0% Dose-dependent; class effect; clinically not significant Clinically significant acute liver injury Very rare May be seen in combination with other medications Fulminant hepatic failure Extremely rare (isolated case reports) It was estimated that risk of fulminant liver failure is 2 per million Autoimmune hepatitis Case reports Statins may induce AIH in genetically susceptible individuals Bhardwaj SS et al. Clin Liver Dis 2007; 11:597-613 Statins and Intracerebral Hemorrhage Meta-analysis of 23 RCTs shows no increased risk Trial 4D ACAPS AFCAPS ALERT ALLHAT ASCOT ASPEN AURORA Bone et al. CARE CLAPT CORONA GISSI-HF GISSI-P GREACE HPS JUPITER LIPID MEGA MIRACL PROSPER SPARCL SSSS Overall RR (95% CI) Weight 0.64 (0.21-1.96) 0.14 (0.01-2.75) 3.00 (0.12-3.62) 0.59 (0.27-1.28) 3.42 (1.26-9.27) 0.55 (0.26-1.14) 1.98 (0.36-10.9) 1.04 (0.57-1.91) 0.74 (0.03-18.3) 0.33 (0.07-1.65) 0.34 (0.01-8.34) 1.66 (0.72-3.80) 3.69 (1.03-13.2) 2.99 (0.12-73.6) 1.00 (0.06-16.0) 0.96 (0.65-1.41) 0.67 (0.24-1.87) 1.89 (0.84-4.24) 1.09 (0.56-2.11) 0.14 (0.01-2.78) 0.81 (0.32-2.04) 1.68 (1.09-2.60) 1.75 (0.51-6.00) 1.10 (0.86-1.42) 3.9 0.7 0.6 6.5 4.7 7.0 1.9 8.8 0.6 2.2 0.6 6.1 3.2 0.6 0.8 12.5 4.4 6.2 7.9 0.7 5.2 11.6 3.4 100 Median LDL-C reduction 1.03 mmol/L Risk ratio for intracerebral hemorrhage 0.01 0.1 1.0 10 100 Statins better Statins worse Hackam DG et al. Circulation 2011; 124:2233-42 Statins and Neuropsychiatric Effects Dementia • Systematic review showed no increased risk of cognitive decline Law et al. Am J Cardiol 2006; 97:52C Suicide / violent death • Conflicting evidence on relationship between statin use mood states: depression, anxiety, fatigue, confusion and vigour While et al. Eur J Cardiovasc Nurs 2010 Insomnia • Initial studies suggested insomnia with lovastatin compared with pravastatin Black et al. JAMA 1990; 264:1105 • Study with objective measures of sleep showed no effect Ehrenberg et al. Sleep 1999; 22:117 Statins and Cognitive Impairment FDA review of case reports, observational data and randomized clinical trials FDA concluded: Some individuals over 50 years old suffer notable, but ill-defined memory loss / impairment Reversible on discontinuation of statin Variable time of onset (1 day to years) No fixed or progressive dementia Not related to specific statin FDA Safety Communication http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm Statins and Fatigue Randomised subset of a controlled trial (397 of 1016 subjects) Equal randomisation to simvastatin 20mg, pravastatin, or placebo 6 month follow-up Table. Change in Energy and Exertional Fatigue Outcome (EnergyFatigueEx) for Placebo vs Statin Groups Placebo All Women Statin Simvastatin Mean ± SD Mean ± SD P value Mean ± SD P value -0.06 ± 0.71 -0.08 ± 0.72 -0.21 ± 0.87 -0.39 ± 1.14 0.005 0.01 -0.25 ± 0.87 -0.47 ± 1.20 0.002 0.004 Pravastatin Mean ± SD P value -0.17 ± 0.86 -0.31 ± 0.72 0.06 0.07 Conclusions: • Statins may worsen energy and or exertional fatigue • Women appear more affected than men • 40% women complain of worse or much worse fatigue However this is a small study of randomized subset, with uncertain reproducibility or importance of metrics used Golomb BA et al. Arch Intern Med. 2012; 172(15):1180-2. doi:10.1001/archinternmed.2012.2171 Statins and New-Onset Diabetes Proportion of patients with new-onset diabetes (%) Study WOSCOPS (N=5974) HPS (N=14,543) ASCOT (N=7773) LIPID (N=7937) CORONA (N=3534) JUPITER (N=17,802) Combined all above (N=57,593) Combined all above except WOSCOPS (N=51,619) Statins Placebo 1.9% 4.6% 3.9% 4.3% 5.6% 3.0% 3.8% 4.0% 2.8% 4.0% 3.5% 4.6% 5.0% 2.4% 3.5% 3.5% Absolute risk of developing DM 0.3-0.5% Note Patient reported diabetes No formal testing for diabetes RR, statin vs placebo 0.69 1.14 1.14 0.95 1.13 1.25 1.06 1.13 95% CI 0.49-0.96 0.98-1.33 0.90-1.43 0.77-1.16 0.86-1.49 1.05-1.49 0.93-1.22 (P=0.38) 1.03-1.23 (P=0.008) Risk factors for Statin associated DM Obesity IFG Elevated TG / HDL Sattar N et al. Lancet 2010; 375:735-42 Statins and New-Onset Diabetes In Context of Reduction of CV Events: 5 Trials Comparing Intensive- to ModerateDose Statin Therapy Cases/Total (%) Incident Diabetes Intensive dose PROVE-IT - TIMI 22, 2004 101/1707 (5.9) A to Z, 2004 65/1768 (3.7) TNT, 2005 418/3798 (11.0) IDEAL, 2005 240/3737 (6.4) SEARCH, 2010 625/5398 (11.6) Moderate dose 99/1688 (5.9) 47/1736 (2.7) 358/3797 (9.4) 209/3724 (5.6) 587/5399 (10.9) Pooled odds ratio 1300/16,344 (8.0) 1.12 (1.04-1.22) 1449/16,408 (8.8) OR (95% CI) 1.01 (0.76-1.34) 1.37 (0.94-2.01) 1.19 (1.02-1.38) 1.15 (0.95-1.40) 1.07 (0.95-1.21) 0.5 1.0 2.0 1.0 Odds ratio (95% CI) 2.0 Incident CVD PROVE-IT - TIMI 22, 2004 A to Z, 2004 TNT, 2005 IDEAL, 2005 SEARCH, 2010 315/1707 (18.4) 212/1768 (12.0) 647/3798 (17.0) 776/3737 (20.8) 1184/5398 (21.9) 355/1688 (21.0) 234/1736 (13.5) 830/3797 (21.9) 917/3724 (24.6) 1214/5399 (22.5) 0.85 (0.72-1.01) 0.87 (0.72-1.07) 0.73 (0.65-0.82) 0.80 (0.72-0.89) 0.97 (0.88-1.06) Pooled odds ratio 3134/16,408 (19.1) 3550/16,344 (21.7) 0.84 (0.75-0.94) 0.5 NTT/yr NNH/yr 155 for CV events 498 for new-onset diabetes Preiss D et al. JAMA 2011; 305:2556-64 Statins and New-Onset Diabetes Treatment of 255 patients with statins results in 1 additional case of diabetes over 4 years Statin treatment prevented of 5.4 vascular events in these 255 patients Benefit of statin treatment exceeds risk Monitor fasting glucose and A1C Muscle Adverse Effects of Statin Major symptom limiting the use of statins Clinical features include • Muscle aches, myalgia, weakness • Stiffness, and cramps • CK not increased in most patients Compromises quality of life Reduces medication adherence Mancini GB et al. CJC 2011; 27:635-662 Statin Intolerance Impact of Adverse Effects on Adherence Survey of 10,138 current or former statin users Muscle related side effects reported in • 60% former users • 25% current users Primary reason for discontinuation was side effects (62%) Cohen JD et al. J Clin Lipid 2012; 6 (3):208-15 Muscle Disease Nomenclature Myalgia • Muscle aches or weakness in absence of CK rise Myositis • Elevated CK in presence of muscle symptoms • No absolute CK cut-off to define elevated Rhabdomyolysis • Pronounced CK elevation (> 10x ULN) with muscle symptoms • May be associated with urine myoglobin and renal dysfunction Note CK elevation can be caused by multiple other causes Hypo or hyper thyroidism Physical exertion Renal dysfunction Seizures Artifactual elevation Trauma Mancini GB et al. CJC 2011; 27:635-662 Rhabdomyolysis and Statins Very rare: 0.7 cases / 100,000 person-years Can also occur in absence of statin therapy Incidence for individual statins (AERS) (1 reported case per number of prescriptions) • Lovastatin 5.2 million • Atorvastatin 23.4 million • Pravastatin 27.1 million • Simvastatin 8.3 million • Cerivastatin 320,000 FDA Adverse Reporting System • Rosuvastatin incidence similar to other statins FDA Advisory 2005 Statin dose-related incidence of rhabdomyolysis • Compared to Atorvastatin 10mg 40 mg HR 3.8 (95% CI 2.3-6.6) 80mg HR 11.3 (95% CI 6.4-20.4) Holbrook A et al. Can J Cardiol 2011; 27:146-51 Incidence of Muscle-Related Symptoms during High-dose Statin Treatment 7924 subjects with hypercholesterolemia Received high dose statins for > 3 months • Atorvastatin 40-80mg ● Fluvastatin ER 80mg • Pravastatin 40mg ● Simvastatin 40-80mg Men : Women 2:1 832 (10.5%) reported muscle related symptoms Incidence related to statin type • Fluvastatin • Pravastatin • Atorvastatin • Simvastatin 5.1% 10.9% 14.9% 18.2% PRIMO Bruckert E et al. Cardiovascular Drugs and Therapy 2005; 19:403-14 Incidence of Muscle-Related Symptoms during High-dose Statin Treatment Onset <1 month to 12 months after start Triggers for muscle related symptoms • Unusual physical exertion • Starting new medication Muscle Pain Location • Widespread 60.1% • “All over” 24.2% • Lower extremities > upper body or trunk Muscle pain • Continuous in 25% • Intermittent in 75% duration minutes – hours PRIMO Bruckert E et al. Cardiovascular Drugs and Therapy 2005; 19:403-14 Clinical Trials and Muscle Related Adverse Effects Meta-analysis of statins vs. placebo studies 18 Studies with 71,108 patients and 301,374 patient-years Muscle related events: • Statin Rx: 316 patients; Placebo 253 patients (P<0.001) CK elevation: • Statin Rx: 81 patients; Placebo 64 (P=0.001) Rhabdomyolysis: • Statin Rx: 10 patients; Placebo 5 patients (NS) NNT = 27 (All cause mortality, MI, CVA, revascularisation) NNH = 3400 (Rhabdomyolysis or CK > 10xULN) Silva M et al. Clin Ther 2006; 29 (2):253-60 Clinical Trials and Muscle-Related Adverse Effects Why the low incidence in clinical trials? Patients highly selected Often have pre-randomisation “run-in” Definition of muscle adverse effects differ Motivated trial patients may minimize symptoms Muscle aches and pains are common in placebo group Mancini GB et al. CJC 2011; 27:635-662 Mechanisms of Statin Myopathy Excess exposure to statins • Increased plasma levels • Increased transmembrane flux of statins Pre-existing neuromuscular disease • May be previously undiagnosed Impaired calcium handling in skeletal muscle Statin induced myocellular metabolic dysfunction Immune mediated inflammatory myopathy • Idiopathic inflammatory myopathy (polymyositis) • Immune mediated necrotizing myopathy Mancini GB et al. CJC 2011; 27:635-662 Risk Factors for Statin Induced Myopathy Patient Characteristics Demographics Older Age, Female gender Asian race Genetic Predisposition Transporters SLCO1B1 CYP isoenzymes FH of statin intolerance Comorbidities Hypothyroidism Systemic disease Alcoholism / drugs Major surgery Myopathy • Hereditary (PYGM, CTP II, AMPD) • Acquired Risk Factors for Statin-Induced Myopathy Statin Dose and Pharmacodynamics Statin dose Muscle-related side effects not related to lipid lowering potency Dose threshold generally above approved doses High vs low statin dose • 7 RCT meta analysis N= 29,395 • No increase in myopathy Properties of statin Bioavailabity Lipophilicity Potential for drug interactions • CYP450 inhibitors • Inhibition of glucuronidation (eg. gemfibrozil) Josan K et al. CMAJ 2008; 178:576-84 Risk Factors for Statin-Induced Myopathy Drug Interactions related to CYP Metabolism CYP 3A4 Simvastatin Lovastatin Atorvastatin CYP 2C9 Fluvastatin Rosuvastatin Inhibitors Protease inhibitors Cyclosporine Amiodarone Fibrates Macrolide antibiotics Diltiazem Inhibitors Cyclosporine No CYP Metabolism Pravastatin Medication Interactions as Cause of Statin-Induced Muscle-Related Side Effects Fibrates • Avoid statin + gemfibrozil • Statin + fenofibrate or bezafibrate can be used cautiously Anti-rejection drugs • Cyclosporine, tacrolimus, sirolimus mycophenylate, rapamycin, 3T3 • Limit statin doses to rosuvastatin 5 mg, atorvastatin 10 mg Antifungals Discontinue statin Macrolide antibiotics during treatment HIV protease inhibitors • Avoid lovastatin and simvastatin • Initiate atorvastatin at 10mg Rare cause of statin myopathy Amiodarone Initiate lower doses of statin Diltiazem Genetics and Statin-Induced Muscle Pains SNP polymorphism rs4149056 of SLCO1B1 gene Encodes transporter for hepatic uptake of statins • For the less common C allele, ORs for simvastatin-induced myopathy (mainly at the 80 mg dose) • heterozygous 4.5 homozygous 16.9 C variant could account for ~60% of statin MRSE Not all C homozygotes develop MRSE Need additional genetic factor • e.g. partial carnitine palmitoyl transferase II (CPTII) deficiency Or medication e.g. fibrate May be most specific for high-dose simvastatin No indication for testing currently Search Collaborative Group. NEJM 2008; 359:789-99 Genetic Risk Factors Associated with Statin-Induced Myopathies Cross sectional study of 136 patients with druginduced myopathies Mutations for metabolic myopathies • Symptomatic 10% vs. control 3%, P=0.04 Increased frequency of carriers vs control • CMT II 13 fold • McArdle disease 20 fold • Myoadenylate deaminase deficiency 3.25 fold Biopsies abnormal in 52% Vladutiu GD et al. Muscle Nerve 2006; 34:153-62 Biochemical Effects of Statins Acetate Acetyl-CoA Acetoacetyl-CoA HMG-CoA Statins Mevalonate Mevalonate-5-P Mevalonate-5-PP Co-enzyme Q10 MVK Protein prenylation Geranylgeranyl-PP Geranyl-PP Farnesyl-PP Squalene Isopentyl-PP Dolichols Squalene-2,3-epoxide Sec-tRNA Lanosterol N-linked glycosylation 7-Dehydrocholesterol Cholestadien-3β-ol Lanosterol DHCR7 DHCR24 *2 Cholesterol Desmosterol *3 Statin Myopathy A Metabolic Muscle Disease ? Proposed Mechanisms Depletion of isoprenoids • Results in reduced protein prenylation • Disruption of small G protein function • Alterations in protein handling • Alterations of gene expression Depletion of coenzyme Q Mitochondrial dysfunction Impaired calcium handling Unmasking pre-existing metabolic myopathies • McArdle disease, CPT II deficiency Prevention of Statin Intolerance Pre-treatment assessment • Assess risk (e.g. elderly, prior muscle pains, FH of myopathy, renal disease, DM, hypothyroidism) • Consider exogenous factors (e.g. statin dose, alcohol use, drug-drug interactions, excessive grapefruit juice use) • Measure baseline CK, ALT, TSH, creatinine Counseling • Inform that statins are very well tolerated in most people • Inform about muscular symptoms and when to discontinue Monitoring • Check CK / ALT when monitoring lipid lowering efficacy At 6-8 weeks after starting or with dose increase and then every 6-12 m Avoid severe exercise for several days prior to testing Therapeutic Options for Management of Statin “Intolerant” Patient Dietary and health behaviour measures Statin based strategies • Alternative statin • Alternative dosing Non-statin alternatives and adjuncts • Ezetimibe • Bile acid sequestrants Fibrates Niacin Dietary and Health Behaviour Measures for Reduction of LDL Cholesterol Reduced dietary fat • Low cholesterol, saturated fat, trans-fats • Low saturated fat • Low trans-fat Replacement diets • Saturated fat replaced with mono or polyunsaturated fats Plant sterols Viscous fibre Red yeast rice – A “LOVASTATIN-LIKE” PRODUCT NCEP Diet Lowers LDL-C Only when Combined with Exercise 10 NCEP Step 2 Diet Cholesterol < 200mg/d <30% calories from fat <7% saturated fat 8 6 4 Change (%) 2 0 -2 -4 -6 -8 -10 -12 -14 Control group Exercise group Diet group Diet-plus-exercise group † ‡ -16 Women Men HDL Cholesterol Women Men LDL Cholesterol Stefanick ML et al. N Engl J Med 1998; 339:12-20 Poly-unsaturated or Mono-unsaturated Fats to Lower LDL-C Baseline mixed natural diet • 19.3% saturated, 11.5 % mono-unsaturated, 4.6% poly-unsaturated Mono-unsaturated Fat Diet (Olive oil + sunflower oil enriched) • 12.9% saturated, 15.1% mono-unsaturated, 7.9% poly-unsaturated Poly-unsaturated Fat Diet (Sunflower oil alone) • 12.6% saturated, 10.8% mono-unsaturated, 12.7% poly-unsaturated LDL-C change Mono-unsaturated diet: Poly-unsaturated diet: - 17.9% - 12.9% Mensink RP et al. New Engl J Med 1989; 321:436-41 Diet and LDL Reduction Portfolio Diet compared to Statins Control Change from baseline (%) LDL-C 25 20 15 10 5 0 -5 -10 -15 -20 -25 -30 -35 -40 -45 0 2 Week Statin Dietary portfolio LDL-C-HDL ratio 4 25 20 15 10 5 0 -5 -10 -15 -20 -25 -30 -35 -40 -45 0 2 Week 4 C-reactive protein 25 20 15 10 5 0 -5 -10 -15 -20 -25 -30 -35 -40 -45 0 2 Week Reduced dietary fat (<30%) and cholesterol (160mg/1000kcal) Increased plant sterols: soy proteins and nuts Increased viscous fibre 4 Jenkins DJ et al. JAMA 2003; 290:502-10 Statin Intolerance Red Rice Yeast Red rice yeast 2400 mg bid vs pravastatin 20 mg bid LDL-C reduction • Red rice yeast 27%, pravastatin 30% NS Myalgia • Red rice yeast 5%, pravastatin 9% NS Halbert et al. Am J Cardiol 2010; 105:198 Variable potency in different preparations, • Not well regulated or standardized Not recommended as replacement for statin Statin based Options for LDL-Cholesterol Lowering in Statin “Intolerant” Patient Lower statin dose Switch to alternative statin Altered dosing regimens • Rosuvastatin 2.5-10 mg 3 x weekly or alternate days • Rosuvastatin 5-20 mg once weekly Low dose / alternative statin /alternating day rosuvastatin + • Ezetimibe • Bile acid binding resin Statin Intolerance Treatment with Low Dose Statins Degreef et al. Eur J Int Med 2010; 21:293 Simvastatin 0.825-8.75mg daily 57% able to tolerate, 30% recurrent muscle pains LDL-C 26% 20% able to tolerate statin achieved LDL-C < 2.5 Glueck et al. Clin Ther 2006; 6:933 61 patients 38-80 years – 50 with myalgia Moderate risk Rx rosuvastatin 5 mg High risk Rx 10mg LDL-C reduction 5 mg -42 + 18 % 10mg -42 + 24% Only 1 /61 unable to tolerate statin Statin Intolerance Reduced Frequency Dosing Alternate day • Retrospective analysis 51 patients (76% myalgia) • Alternate day rosuvastatin (mean dose 5.6mg) 72.5% able to tolerate LDL-C reduced 34.5% P<0.001 in patients tolerating statin Backes JM et al. Ann Phamacotherapy 2008; 42:341-46 • Two patients intolerant of atorvastatin • Changed to rosuvastatin 2.5-5mg 3x / week Well tolerated LDL-C reduced 20-38% Mackie BD et al. Am J Cardiol 2007; 99:291 Once weekly • 10 patients (7 myalgias; 3 GI complaint on prior statin) • Weekly rosuvastatin 5-20 mg • LDL-C reduced 29% (range 6-62%) • 2 patients discontinued because of similar symptoms Backes JM et al. Am J Cardiol 2007; 100:554-55 Fluvastatin, Ezetimibe or Both in the Management of Statin Intolerance 199 patients with statin Intolerance Received either Fluvastatin XL 80mg or Ezetimibe 10mg or Fluvastatin + Ezetimibe 97% tolerated treatment 17% develop tolerable muscle symptoms 200 LDL-C (mg/dl) 180 160 Ezetimibe -15.6% 140 120 Fluvastatin XL -32.8% 100 -46.1% Fluvastatin/Ezetimibe 80 60 0 4 8 Time (weeks) 12 Stein EA et al. Am J Cardiol 2008; 101:490-96 Ezetimibe / Fluvastatin XL or Combination in Statin Intolerant Patients Time to Onset of Muscle Related Side Effects 30 Ezetimibe 25 20 HR 0.73, 95% CI 0.35-1.55, P=0.41 Fluvastatin XL HR 0.52, 95% CI 0.23-1.19, P=0.12 15 Fluvastatin/Ezetimibe 10 5 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Time since randomization (weeks) Stein EA et al. Am J Cardiol 2008; 101:490-96 Coenzyme Q10 and Statin Myopathy HMG-CoA Reductase Inhibitors (Statins) Squalene Cholesterol Caso et al. Am J Cardiol 2007; 99:1409 • 32 patients HMG-CoA reductase • Myopathic symptoms Mevalonate • Co Q10 100mg or Vit E for 30 days • Pain severity 40% (P<0.001) Isopentenyl Young et al. Am J Cardiol 2007; 100:1400 Pyrophosphate • 44 patients statin intolerant • Co Q10 200 mg or placebo for 12 weeks Farnesyl Pyrophosphate during simvastatin titration 10 to 40 mg daily Geranylgeranyl • No difference in proportion Pyrophosphate Able to tolerate simva 40 mg Prenylation Prenylation Remaining on statin HMG-CoA Isoprenylated Proteins Ubiquinone Systematic Review: Insufficient evidence to support any role for Coenzyme Q10 in the management or prevention of statin related myopathy Marcoff L and Thompson PB. JACC 2007; 49:2231-7 Non-Statin Lipid Lowering Strategies Ezetimibe Lowers LDL 15-20% Well tolerated May be added to low dose statin Bile acid sequestrants Lowers LDL 15% May prevent diabetes Colesevalam better tolerated Fibrates TG LDL little change ? Benefit when HDL low Niacin Flushing/pruritus may limit tolerance Lowers LDL 20% TG 40%, HDL 30% Ezetimibe + Bile acid sequestrant 40-45% LDL reduction Future Non-Statin Strategies to Reduce LDL Cholesterol CETP inhibitor • Torcetrapib (increased mortality) and Dalcetrapib (no benefit) • Anacetrapib results awaited ( HDL 138%, LDL 40%) • Evacetrapib Phase 2 study presented 2010 Mipomersen • Inhibits protein synthesis of apoB • Reduces LDL ~30% • Injected weekly • No outcomes trials PCSK9 inhibitors • Reduce LDL 50-60 %, • Injected q 2 weeks • No outcomes trials Adherence to Lipid Lowering Therapy and Outcomes Adherence rates at 1 year 26-85% Muscle related symptoms more frequent in non-adherent patients Patient perception of short-term disadvantages outweighs any long-term benefits Outcomes worse in non-adherent patients RRR for CV event according to adherence RRR % • Fully adherent 39.3% • 50% adherent 26.1% • 25% adherent 10.9% Lipid Research Clinic. Miller NH Am J Med 1997; 102 Suppl 1:43-49 Determinants of Non-Adherence to Statin Therapy Younger patient age Primary vs. secondary prevention Cost (copayment by patient) Not taking other medication Male gender No diabetes nor hypertension No recent acute MI (vs. chronic CAD) Infrequent physician visits Adverse effects of medication Algorithm for Statin-Induced Myopathy Symptoms of Muscle Pains or Asymptomatic Elevation of CK CK not elevated CK < 10x ULN CK >10x ULN With significant symptoms D/C statin D/C statin D/C statin Restart when free of symptoms Consider precipitating ff Consider precipitating ff eg thyroid, exercise, drug interactions Check creatinine, urine for myoglobin Follow until CK normal Hydrate Reassess CK 6-12 weeks later or if symptoms reoccur Restart statin lower dose Use statin with less MRE e.g. rosuvastatin, fluvastatin Consider low dose statin, non-statin options Use dietary measures Conclusions Adverse Effects of Statin Treatment More common than clinical trials suggest Probably more frequent at higher doses Important cause of poor adherence to treatment Prevent statin induced muscle adverse events Manage adverse events • Use alternative statin • Reduce frequency of statin • Use non-statin agents as monotherapy or together with reduced dose or frequency statin Back-up Slide Effect of Statins on CV Event Rates Related to 1 mmol/l LDL Reduction A. More statin vs less statin (5 trials: 0.51 mmol/L LDL difference) Any major coronary event 1725 (1.9) Any coronary revascularization 2250 (2.6) Any stroke 572 (0.6) 5 trials: any major vascular event 3837 (4.5) B. Statin vs control (21 trials: 1.07 mmol/L LDL difference) Any major coronary event 3380 (1.3) Any coronary revascularization 3103 (1.2) Any stroke 1730 (0.7) 21 trials: any major vascular event 7136 (2.8) C. More statin vs less statin vs control (26 trials) Vascular events Major vascular event Patients with type 1 diabetes 145 (4.5) Patients with type 2 diabetes 2494 (4.2) Patients without diabetes 8272 (3.2) Any vascular event 10,973 (3.2) Mortality Cause-specific mortality All cardiac 3333 (0.9) Stroke 483 (0.1) Any vascular 4220 (1.2) Any non-vascular 2943 (0.8) All-cause mortality (any death) 7642 (2.1) Relative risk per 1 mmol/L (39 mg/dl) reduction in LDL-C (95% CI) 0.74 1973 (0.65-0.85) (2.2) 0.66 2741 (0.60-0.73) (3.2) 0.74 (0.59-0.92) 663 (0.7) 0.72 4416 (0.66-0.78) (5.3) More statin better Less statin better 0.76 4539 (0.73-0.79) (1.7) 0.76 4066 (0.73-0.80) (1.6) 0.85 2017 (0.80-0.90) (0.8) 0.79 8934 (0.77-0.81) (3.6) Statin better Control better 0.77 (0.58-1.01) 192 (6.0) 0.80 2920 (0.74-0.86) (5.1) 0.78 10,163 (0.75-0.81) (4.0) 0.78 13,350 (0.76-0.80) (4.0) 0.84 3384 (0.80-0.88) (1.1) 0.96 (0.84-1.09) 501 (0.1) 0.86 4220 (0.82-0.90) (1.2) 0.92 2994 (0.92-1.03) (0.8) 0.90 8327 (0.87-0.93) (2.3) 0.50 0.75 1.00 1.25 1.50 Statin or more statin better Control or less statin better CCT Trialists. Lancet 2010;376:1670