* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Plavix® Consultants Kit Blue
Survey
Document related concepts
Cardiovascular disease wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Drug-eluting stent wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Coronary artery disease wikipedia , lookup
Transcript
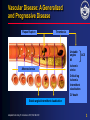
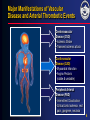
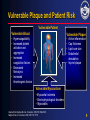
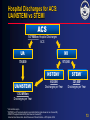
ACS* Joseph G. Cacchione, M.D. December 9, 2005 * UA/NSTEMI=unstable angina/non–ST-segment elevation myocardial infarction (also known as non–Q-wave MI). 1 What is the pathophysiology of arterial thrombosis? 2 Vascular Disease: A Generalized and Progressive Disease Plaque Rupture Thrombosis Unstable angina MI Atherosclerosis ACS Ischemic stroke Critical leg ischemia Intermittent claudication CV death Stable angina/intermittent claudication Adapted from Libby P. Circulation. 2001;104:365-372. 3 Major Manifestations of Vascular Disease and Arterial Thrombotic Events Cerebrovascular Disease (CVD) • Ischemic Stroke • Transient ischemic attack Cardiovascular Disease (CAD) • Myocardial infarction • Angina Pectoris (stable & unstable) Peripheral Arterial Disease (PAD) • Intermittent Claudication • Critical Limb Ischemia, rest pain, gangrene, necrosis 4 What are the risk factors associated with ACS*? * UA/NSTEMI=unstable angina/non–ST-segment elevation myocardial infarction (also known as non–Q-wave MI). 5 Identifying Those at Risk of Vascular Disease and Thrombotic Events Lifestyle • Smoking • Diet • Lack of exercise Genetic Traits • Gender • Age Generalized Disorders • Obesity • Diabetes Arterial Thrombosis Manifestations (MI, stroke, vascular death) Inflammation • Elevated CRP • Prothrombotic factors • Fibrinogen • IL6 and others CRP=C-reactive protein. Yusuf S et al. Circulation. 2001;104:2746-2753. Drouet L. Cerebrovasc Dis. 2002;13(suppl 1):1-6. Systemic Conditions • History of vascular events • Hypertension • Hyperlipidemia • Hypercoagulable states • Homocysteinemia Local Factors • Blood flow patterns • Shear stress • Vessel diameter • Arterial wall structure • % arterial stenosis 6 What role does “vulnerable plaque” play in ACS*? * UA/NSTEMI=unstable angina/non–ST-segment elevation myocardial infarction (also known as non–Q-wave MI). 7 Characteristics of Unstable (Vulnerable) and Stable Plaques Unstable Stable Inflammatory Cells Few SMCs Thin Fibrous Cap More SMCs Thick Fibrous Cap Lack of Inflammatory Cells Intact Endothelium Eroded Endothelium Activated Macrophages SMC=smooth muscle cell. Adapted from Libby P, et al. Circulation. 1995;91:2844-2850. Naghavi M et al. Circulation. 2003;108:1664-1672. Foam Cells 8 Vulnerable Plaque and Patient Risk Vulnerable Patient Vulnerable Blood Vulnerable Plaque • Hypercoagulability • Increased platelet activation and aggregation • Increased coagulation factors • Decreased fibrinolysis • Increased thrombogenic factors • • • • Active inflammation Cap thickness Lipid core size Endothelial denudation • Injured plaque Vulnerable Myocardium • Myocardial ischemia • Electrophysiological disorders • Myocarditis Adapted from Naghavi M et al. Circulation. 2003;108:1664-1672. Naghavi M et al. Circulation. 2003;108:1772-1778. 9 What is the epidemiology of ACS*? * UA/NSTEMI=unstable angina/non–ST-segment elevation myocardial infarction (also known as non–Q-wave MI). 10 Arterial Thrombosis* Is a Leading Cause of Death Worldwide1† Leading Causes of Death Worldwide† ( % of All Deaths)1 AIDS Pulmonary disease Injuries Cancer Infectious disease 5.1% 6.0% 9.1% 12.6% 17.8% Arterial thrombosis* 23% Mortality (%) * Ischemic heart disease and cerebrovascular disease. † Worldwide defined as Member States by WHO Region (Africa, Americas, Eastern Mediterranean, European, South-East Asia and Western Pacific). 1. The World Health Report, 2002, WHO Geneva. 11 Ongoing Risk of MI and Stroke Over 10 Years Post-MI Increased risk of MI* Increased risk of stroke* 5-7 3-4 greater risk1 (includes death) 2-3 Post-Stroke greater risk2 (includes angina and sudden death†) 4 PAD greater risk4 (includes only fatal MI and other CHD death) greater risk2 (includes TIA) 9 greater risk3 2-3 greater risk2 (includes TIA) * Versus the general population except for stroke following stroke which measures subsequent risk per year. † Sudden death defined as death documented within 1 hour and attributed to coronary heart disease. 1. Adult Treatment Panel II. Circulation. 1994;89:1333-1435. 2. Kannel WB. J Cardiovasc Risk. 1994;1:333-339. 3. Wilterdink JI, Easton JD. Arch Neurol. 1992;49:857-863. 4. Criqui MH, et al. N Engl J Med. 1992;326:381-386. 12 Hospital Discharges for ACS: UA/NSTEMI vs STEMI ACS 1.67 Million Hospital Discharges ACS UA* MI 700,000 973,000 UA/NSTEMI NSTEMI† STEMI** 652,000 Discharges per Year 321,000‡ Discharges per Year 1.352 Million Discharges per Year * UA=unstable angina. † NSTEMI=nonST-segment elevation myocardial infarction (also known as non–Q-wave MI). **STEMI=ST-segment elevation MI (also known as Q-wave MI). American Heart Association. Heart Disease and Stroke Statistics—2005 Update. 2004. 13 What are the goals of ACS* management? • Acute management? • Long-term management? * UA/NSTEMI=unstable angina/non–ST-segment elevation myocardial infarction (also known as non–Q-wave MI). 14 What are the goals of ACS* management? • Acute management? • Long-term management? * UA/NSTEMI=unstable angina/non–ST-segment elevation myocardial infarction (also known as non–Q-wave MI). 15 Brigham and Women’s Hospital Critical Pathway 2002: UA/NSTEMI Aspirin, Clopidogrel, UFH or LMWH Beta-blocker, Nitrates Low Risk High Risk – ECG, – Markers TIMI Risk Score <3 ST s, +Troponin/CK-MB, or TIMI Risk Score >3 No Cath Planned Cath GP IIb/IIIa Inhibitor ETT Cath/PCI (on GP IIb/IIIa ) Cath GP IIb/IIIa for PCI D/C Day 1 or 2 D/C Day 2 D/C Day 2 ETT=exercise tolerance test. Modified from Cannon CP. Crit Path Cardiol. 2002;1:12-21 with permission of the author. 16 What are the goals of ACS* management? • Acute management? • Long-term management? * UA/NSTEMI=unstable angina/non–ST-segment elevation myocardial infarction (also known as non–Q-wave MI). 17 Distribution of Multiple Plaques in Patients with Acute Coronary Syndromes 80% of patients with >1 plaque N=24 30 Patients (%) 25 20 15 10 5 0 0 1 2 3 4 5 Number of ruptured plaques in addition to culprit lesion Rioufol G et al. Circulation. 2002;106:804-808. 18 An Integrated Approach to Managing Patients With Acute Coronary Syndrome* • Focal interventional treatment to stabilize ruptured plaque – PCI with stent • Systemic medical therapy intended to stabilize plaque and inhibit clot formation, thus helping to reduce the risk of thrombotic events (MI, stroke, or death) due to diffuse atherosclerosis – aspirin, clopidogrel, lipid-lowering therapy, ACE inhibitors, beta-blockers * ACS=unstable angina/non–Q-wave MI. Ambrose JA. Circulation. 2002;105:2000-2004. 19 What evidence is there to support the use of clopidogrel in ACS*? * UA/NSTEMI=unstable angina/non–ST-segment elevation myocardial infarction (also known as non–Q-wave MI). 20 Mechanisms of Action of Oral Antiplatelet Therapies ADP clopidogrel bisulfate ticlopidine HCl dipyridamole ADP phosphodiesterase ADP cAMP Activation GP IIb/IIIa (fibrinogen receptor) collagen thrombin TXA2 COX TXA2 aspirin ADP=adenosine diphosphate, TXA2=thromboxane A2, COX=cyclooxygenase. Schafer AI. Am J Med. 1996;101:199-209. 21 Plavix® (clopidogrel bisulfate) Inhibits a Key Step in Thrombus Formation Adapted from Libby P. Circulation. 2001;104:365-372; Frishman WH et. al. Am J Cardiol. 1990;66:66G-70G. 22 Antithrombotic Trialists’ Collaboration Efficacy of Aspirin Doses on Vascular Events in High-Risk Patients Aspirin Dose # Trials 500–1500 mg 34 19 160–325 mg 19 26 75–150 mg 12 32 3 13 65 23 <75 mg Any aspirin Odds Ratio OR* (%) 0 0.5 Antiplatelet Better 1.0 1.5 2.0 Antiplatelet Worse * Odds reduction. Treatment effect P<0.0001. Adapted with permission from the BMJ Publishing Group. Antithrombotic Trialists’ Collaboration. BMJ. 2002;324:71-86. 23 CURE CURE: Clopidogrel in Unstable Angina to Prevent Recurrent Events Loading dose Maintenance dose clopidogrel 300 mg + ASA 75–325 mg clopidogrel 75 mg + ASA 75–325 mg placebo + ASA 75–325 mg placebo + ASA 75–325 mg N=12,562 n=6,259 Patient Population • Patients with ACS presenting within 24 hours n=6,303 Primary End Point • Composite of death from cardiovascular causes, nonfatal MI, or stroke CURE Trial Investigators. N Engl J Med. 2001;345:494-502. 482 centers 28 countries 24 CURE Primary End Point: MI/Stroke/CV Death 0.14 0.12 Cumulative Hazard Rate 20% Placebo + ASA* Relative Risk Reduction 0.10 P=0.00009† N=12,562 0.08 Clopidogrel + ASA* 0.06 The primary outcome occurred in 9.3% of patients in the clopidogrel + ASA group and 11.4% in the placebo + ASA group. 0.04 0.02 0.00 0 3 6 9 12 Months of Follow-Up * Other standard therapies were used as appropriate. † PLAVIX Prescribing Information. Adapted with permission (2002) from the Massachusetts Medical Society. The CURE Trial Investigators. N Engl J Med. 2001;345:494-502. 25 CURE Patients Treated with Medical Therapy Cumulative Hazard Rate 0.20 Without PCI* and/or CABG 0.15 Placebo + ASA† (10.0%) 20% Relative Risk Reduction 0.10 Clopidogrel + ASA† 0.05 (P=0.0025‡) (8.1%) 0.00 0 100 200 300 Days of Follow-Up * PCI was also referred to as PTCA. † Other standard therapies were used as appropriate. ‡ In the combined end point of MI, stroke, or CV death. Only first events after randomization were counted in the composite end point. Data on file, Sanofi-Synthelabo Inc. 26 CURE Patients Treated with PCI* and/or CABG Placebo + ASA† 0.20 18% Relative Risk Reduction Cumulative Hazard Rate (13.8%) 0.15 (P=0.015‡) Clopidogrel + ASA† 0.10 (11.4%) 0.05 0.00 0 100 200 300 Days of Follow-Up * PCI was also referred to as PTCA. † Other standard therapies were used as appropriate. ‡ In the combined end point of MI, stroke, or CV death. Only first events after randomization were counted in the composite end point. Data on file, Sanofi-Synthelabo Inc. 27 CURE Main Efficacy Results: Combined Co-primary End Points Outcome Clopidogrel + ASA* Placebo + ASA* (n=6,259) (n=6,303) Primary outcome Relative Risk Reduction P value 9.3% 11.4% 20% 0.00009 16.5% 18.8% 14% 0.0005 5.2% 6.6% 23% 1.2% 1.4% 14% 5.1% 5.5% 7% 8.7% 9.3% 7% (MI, stroke, CV death) Co-primary outcome† All individual outcome events‡: MI Stroke CV death Refractory ischemia * Other standard therapies were used as appropriate. † MI, stroke, CV death, or refractory ischemia. ‡ The individual components represent the total number of subjects experiencing an event during the course of the study. PLAVIX Prescribing Information. 28 CURE Beneficial Outcomes with Clopidogrel in Various Subgroups Percentage of Patients With Event Characteristic Overall No. of Patients Clopidogrel + ASA* Placebo + ASA* 12562 9.3 11.4 Male sex Female sex 7726 4836 9.1 9.5 11.9 10.7 < 65 yr old > 65 yr old 6354 6208 5.4 13.3 7.6 15.3 ST-segment deviation No ST-segment deviation 6275 6287 11.5 7.0 14.3 8.6 Diabetes No diabetes 2840 9722 14.2 7.9 16.7 9.9 0.4 0.6 0.8 1.0 1.2 Clopidogrel Better Placebo Better Relative Risk (95% CI) * In addition to other standard therapies. The CURE Trial Investigators. N Engl J Med. 2001;345:494-502. 29 CURE Outcomes With Clopidogrel in Addition to Standard Therapy Concomitant Medication Heparin/LMWH No Yes Aspirin N Clopidogrel + ASA* Placebo +ASA* 951 11611 4.9 9.7 7.7 11.7 <100 mg 100-200 mg 1927 7428 8.5 9.2 9.7 10.9 >200 mg 3201 9.9 13.7 IV GP IIb/IIIa† No Yes 11739 823 8.9 15.7 10.8 19.2 Beta-blockers No Yes 2032 10530 9.9 9.2 12.0 11.3 ACE Inhibitors No Yes 4813 7749 6.3 11.2 8.1 13.5 Lipid-lowering No Yes 4461 8101 10.9 8.4 13.1 10.5 PTCA/CABG No Yes 7977 4585 8.1 11.4 10.0 13.8 0.4 * Other standard therapies were used as appropriate. † Use not permitted within 3 days prior to randomization. PLAVIX Prescribing Information. 0.6 1.2 0.8 1.0 Clopidogrel Placebo Better Better Relative Risk (95% CI) 30 CURE Bleeding Results Clopidogrel + ASA* Placebo + ASA* (n=6,259) (n=6,303) P value 3.7% 2.7% 0.001 Life-threatening bleeding 2.2% 1.8% 0.13 Other major bleeding 1.6% 1.0% 0.005 Minor bleeding 5.1% 2.4% <0.001 Event Major bleeding† * Other standard therapies were used as appropriate. † Life-threatening and other major bleeding. PLAVIX Prescribing Information. 31 CURE Major Bleeding by ASA Dose ASA Dose Clopidogrel + ASA* Placebo + ASA* <100 mg 2.6% 2.0% 100–200 mg 3.5% 2.3% >200 mg 4.9% 4.0% * Other standard therapies were used as appropriate. PLAVIX Prescribing Information. 32 What are the 2002 ACC/AHA UA/NSTEMI* guideline recommendations for clopidogrel therapy? * UA/NSTEMI=unstable angina/non–ST-segment elevation myocardial infarction (also known as non–Q-wave MI). 33 2002 ACC/AHA UA/NSTEMI* Guideline Update: Recommendations for Long-Term Medical Therapy Class I Aspirin 75 to 325 mg/day (level of evidence: A) Clopidogrel 75 mg daily (in the absence of contraindications) when ASA is not tolerated because of hypersensitivity or gastrointestinal intolerance (level of evidence: A) The combination of ASA and clopidogrel for 9 months after UA/NSTEMI (level of evidence: B) Beta-blockers in the absence of contraindications (level of evidence: B) Lipid-lowering agents and diet in post-ACS and post-revascularization patients with LDL cholesterol >130 mg/dL (level of evidence: A) Lipid-lowering agents if LDL cholesterol level after diet is >100 mg/dL (level of evidence: C) ACE inhibitors for patients with CHF, LV dysfunction (EF <0.40), hypertension, or diabetes (level of evidence: A) * Also known as non–Q-wave MI. Braunwald E, et al. Available at: www.acc.org. Accessed February 18, 2004. 34 2002 ACC/AHA UA/NSTEMI* Guidelines Update: Key Recommendations for Clopidogrel Therapy Class I In patients in whom an early noninterventional approach is planned, clopidogrel should be added to ASA as soon as possible on admission and administered for at least 1 month (A) and for up to 9 months (B) In patients in whom a PCI is planned, clopidogrel should be started and continued for at least 1 month (A) and up to 9 months in patients who are not at high risk for bleeding (B) For long-term medical therapy, the combination of ASA and clopidogrel is recommended for 9 months after UA/NSTEMI (B) In patients taking clopidogrel in whom elective CABG is planned, the drug should be withheld for 5 to 7 days (B) * Also known as non–Q-wave MI. Braunwald E, et al. Available at: http://www.acc.org. Accessed February 18, 2004. 35 2002 ACC/AHA UA/NSTEMI* Guidelines Update: Recommendations for Clopidogrel in Long-term Medical Therapy PATIENT TYPE RECOMMENDED THERAPY For patients not receiving PCI Clopidogrel bisulfate with aspirin as soon as possible on admission LENGTH OF THERAPY Administer for at least 1 month CLASS/LEVEL OF EVIDENCE Class I Level A May be administered for up to 9 months Class I Level B For patients receiving PCI with or without stent Clopidogrel bisulfate with aspirin Administer for at least 1 month May be administered for up to 9 months in patients who are not at high risk of bleeding Class I Level A Class I Level B In patients taking clopidogrel bisulfate in whom elective CABG is planned, the drug should be withheld for 5 to 7 days (Level of Evidence: B) * UA/NSTEMI=unstable angina/non–ST-segment elevation myocardial infarction (also known as non–Q-wave MI). Braunwald E, et al. Available at: www.acc.org. Accessed February 10, 2005. 36 ACC/AHA Treatment Recommendations for the Long-term Management of ACS* ACS (UA/NSTEMI* patients) Medical Management Cath lab Medical Management (no intervention required) PCI (with or without stent) CABG† Long-term management 1. 2. 3. 4. 5. Clopidogrel ASA ß-blocker ACE Inhibitor Statin * UA/NSTEMI=unstable angina/non–ST-segment elevation myocardial infarction (also known as non–Q-wave MI). † If possible, withhold clopidogrel 5 to 7 days prior to the procedure. Braunwald E, et al. Available at: www.acc.org. Accessed February 10, 2005. 37 What quality of care programs exist for ACS*? * UA/NSTEMI=unstable angina/non–ST-segment elevation myocardial infarction (also known as non–Q-wave MI). 38 Overview of CRUSADE Quality Improvement Program CRUSADE Quality Improvement Program 1. Implement quality improvement initiatives to promote ACC/AHA NSTE ACS Guidelines recommendations Objectives 2. Improve clinical outcomes for NSTE ACS patients via early risk stratification and implementation of evidence-based care, both in-hospital and post-discharge Up to 600 participating hospitals—a collaborative effort between Emergency Medicine, Cardiology, Hospital QI, Academia, and Industry Participants Methods – Collect data regarding patient management practice patterns in the US – Use those data to target educational interventions designed to promote adherence to the revised ACC/AHA NSTE ACS guidelines recommendations CRUSADE Overview. Available at: http://www.crusadeqi.com. Accessed March 13, 2005. CRUSADE is funded by Millennium Pharmaceuticals, Inc., Schering Corporation, and the Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership. 39 CRUSADE Acute and Discharge Clopidogrel Use by Treatment Approach 96 Utilization of Therapies (%) 100 75 57 45 50 33 26 25 0 Early Cath No Early Cath PCI Acute Therapy Medical Rx CABG Discharge Therapy (clopidodegrel given within 24 hrs of admission) Q4 2004 CRUSADE data. Adapted with permission from CRUSADE Web site. Available at: http://www.crusadeqi.com. Accessed March 11, 2005. 40 CRUSADE Discharge Medication Use – Last 12 Months (In patients without contraindications) 100% 93% 89% 86% Utilization of Therapies (%) 80% 69% 64% 60% 40% 20% 0% ASA Beta Blockers ACEInhibitors* Any LipidLowering Agent† Clopidogrel * LVEF <40%, CHF, DM, HTN. † Known hyperlipidemia, TC, LDL. CRUSADE data October 1, 2003-September 30, 2004 (n=40,386) Adapted with permission from CRUSADE Web site. Available at: http://www.crusadeqi.com. Accessed March 11, 2005. 41 CRUSADE Paradoxical Discharge Care Patterns Patients Treated (%) 100 92 91.9 Low-Risk Moderate-Risk High-Risk 87.9 n=74,217 84 84.1 82.8 70.3 68.7 75 61.7 60.3 59.6 61 58.5 56.6 47.4 50 25 0 ASA Clopidogrel Beta Blocker Adapted with permission from CRUSADE Web site. Available at: http://www.crusadeqi.com. Accessed March 11, 2005. ACE-I Statin 42 CRUSADE Trends in Discharge Therapy Adherence 100% 91% 93% 86% Q4-03 Q1-04 Q2-04 Q3-04 89% 86% 81% 75% 69% 59% 63% 64% 50% 25% 0% Aspirin Clopidogrel Beta blocker ACE Inhibitor CRUSADE data Quarter 4, 2003 through Quarter 3, 2004. Adapted with permission from CRUSADE Web site. Available at: http://www.crusadeqi.com. Accessed March 11, 2005. Lipidlowering Agent 43 CRUSADE Relationship Between Guidelines Adherence and In-Hospital Mortality Improved Hospital Adherence 7 In-Hospital Mortality (%)* 6 6.0% 5.2% 5 5.0% 4.2% 4 3 2 1 0 ≤25% 25–50% 50–75% ≥75% Hospital Composite Adherence Quartiles * Adjusted figure. Cumulative CRUSADE data (adjusted) through September 2004. Adapted with permission from CRUSADE Web site. Available at: http://www.crusadeqi.com. Accessed February 10, 2005. 44 Summary • The American Heart Association estimates that 1.4 million patients are diagnosed with ACS each year • Patients with ACS are at risk for other types of arterial thrombosis, including stroke and PAD • The CURE study showed a 20% relative risk reduction in clopidogrel plus aspirin vs aspirin alone in the combined end point of MI, stroke, or vascular death • The 2002 ACC/AHA UA/NSTEMI* Guidelines for Long-term Management of ACS recommend that clopidogrel may be given daily for up to 9 months • Quality improvement programs increase adherence to guideline-recommended therapies and reduce in-hospital mortality * UA/NSTEMI=unstable angina/non–ST-segment elevation myocardial infarction (also known as non–Q-wave MI). 45 Case: Mrs. Charlotte Purdue* • 72-year-old female who presents to clinic with complaints of episodic chest discomfort – PMH of MI 3 years ago and HTN controlled with a diuretic – She describes the pain as epigastric, sometimes on her left side, and has lasted for 7 days – The pain lasted 2 hours this morning, but she is currently pain-free • Plan: direct admit to the hospital for rule out of ACS† and observation * Patients’ names are fictitious. † UA/NSTEMI=unstable angina/non-ST-segment elevation myocardial infarction (also known as non-Q-wave MI). 46 Case: Mrs. Charlotte Purdue Past Medical History • MI (3 years ago) • Hypertension Medications – Aspirin 81 mg – Diuretic – Statin Physical Examination • • • • • • • WT: 165 lb BP: 158/88 mm Hg P: 80 bpm RR: 18 Lung sounds: clear Cardiac exam: nl S1, S2, +S4 Distal pulses: normal 47 Case: Mrs. Charlotte Purdue • Mrs. Purdue is admitted to the telemetry floor • Her initial ECG shows small T wave inversions in leads II and III, and initial cardiac markers are negative. These are new findings when compared to an old ECG • Working diagnosis: unstable angina Nursing Orders 1. Rule out with cardiac markers Q8 2. Serial ECGs Medication Orders • • • • • • Clopidogrel 300 mg ASA 325 mg Heparin IV β-Blocker Statin Sublingual NTG prn 48 Case: Mrs. Charlotte Purdue • The next morning, Mrs. Purdue continues to be chest pain free. There were no elevations in cardiac markers, and her ECG findings resolve • She is sent to the stress lab for a stress myocardial perfusion scan – Findings: small fixed defect with mild inferolateral ischemia – Normal LVEF (55%) Final diagnosis: unstable angina 49