* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download atorvastatin - Clinical Trial Results
Survey
Document related concepts
Transcript
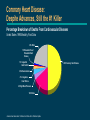
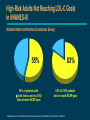
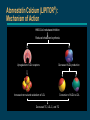
Advances in Lipid Management The National Cholesterol Education Program (NCEP) Launched by National Heart, Lung, and Blood Institute (NHLBI), a part of the NIH, in November, 1985 Impetus: Definitive evidence linking coronary heart disease (CHD) to elevated total cholesterol levels Goal: Educating, monitoring, and developing guidelines for lowering blood cholesterol levels NCEP web site. Coronary Heart Disease: Despite Advances, Still the #1 Killer Percentage Breakdown of Deaths From Cardiovascular Diseases United States: 1995 Mortality, Final Data 22% Other 1% Rheumatic Fever/ Rheumatic Heart Disease 1% Congenital Heart Defects 50% Coronary Heart Disease 2% Atherosclerosis 4% Congestive Heart Failure 4% High Blood Pressure 16% Stroke American Heart Association. 1998 Heart and Stroke Facts: Statistical Update. Major Risk Factors for CHD The NCEP Adult Treatment Panel Identifies Positive Risk Factors (RF) for CHD Risk Factors Family history of early CHD — parent or sibling <55 years of age if male, <65 years of age if female Age — male 45 years — female 55 years, or premature menopause without estrogen replacement therapy (ERT) Hypertensive (BP 140/90 mm/Hg or taking antihypertensive medication) Current smoker Diabetes mellitus Low HDL-cholesterol (<35 mg/dL) Negative Risk Factor If HDL-C is 60 mg/dL, subtract one risk factor Risk Stratification for Adults Without CHD Classification Based on LDL-C LDL-C Level Classification <130 mg/dL Desirable 130-159 mg/dL Borderline-high risk 160 mg/dL High risk NCEP Primary CHD Risk Categories and Goals for Lowering LDL-C Risk Category LDL-C Goal No CHD <2 RF <160 mg/dL No CHD 2 RF <130 mg/dL CHD 100 mg/dL The NCEP recommends lowering LDL-C even further than these goals, if possible. High-Risk Adults Not Reaching LDL-C Goals in NHANES-III (National Health and Nutrition Examination Survey) 55% 55% of patients with 2 risk factors and no CHD did not reach NCEP goal 83% 83% of CHD patients did not reach NCEP goal Unpublished data from the Third National Health and Nutrition Examination Survey (NHANES-III), CDC 1994; data from 1988–1991. Lipid Treatment Assessment Project (L-TAP) Hypothesis — majority of dyslipidemic patients do not achieve NCEP target LDL-C levels Primary Objective — to determine percentage of primary care patients on diet and/or drug therapy who are achieving NCEP LDL-C goals Pearson TA, Laurora IM. Scientific Sessions of the American Heart Association; 1997; Abstract 361. L-TAP: % of Patients at LDL-C Goal Risk group— Lipid-lowering therapy LDL-C goal % Success % Failure Diet therapy 2RF (n=282) 2RF (n=361) CHD (n=108) Total (n=751) 59 22 7 34 41 78 93 66 Drug therapy 2RF (n= 861) 2RF (n=1924) CHD (n=1352) Total (n=4137) 70 40 18 39 30 60 82 61 Does not include patients who were nonevaluable. Person’s chi-square=682.91; d=2; P=0.001. Data on file. Parke-Davis; Morris Plains, NJ. Pearson TA, Laurora IM. Scientific Sessions of the American Heart Association; 1997; Abstract 361. P-Value 0.001 0.001 L-TAP: Identifying the Patient at Risk Patient Profile With 2 Risk Factors 92% Male 45 years CHD Patients 30% No CHD <2 RF No CHD 2 RF 47% 23% Data on file. Parke-Davis; Morris Plains, NJ. Female 55 years 70% Hypertensive (140/90 mm Hg) 41% Family history of early CHD 22% Low HDL-C level 21% Diabetes mellitus 19% Current smokers L-TAP: Many High-Risk Adults Are Not Reaching LDL-C Goals 63% 63% of patients with 2 risk factors and no CHD did not reach NCEP goal Data on file. Parke-Davis; Morris Plains, NJ. 82% 82% of CHD patients did not reach NCEP goal L-TAP: Distance From LDL-C Goal in Patients With 2 Risk Factors 63% Not at goal No. of patients 1000 900 800 700 600 500 400 300 200 100 0 At goal n=849 n=816 n=494 n=126 <130 130-160 161-200 LDL-C (mg/dL) Data on file. Parke-Davis; Morris Plains, NJ. >200 L-TAP: Distance From LDL-C Goal in Patients With CHD 82% Not at goal 600 n=545 500 No. of patients n=416 400 At goal 300 n=256 n=243 200 100 0 100 101-130 131-160 LDL-C (mg/dL) Data on file. Parke-Davis; Morris Plains, NJ. >160 Relationship Between Cholesterol and CHD Risk: Epidemiologic Trials Multiple Risk Factor Intervention Trial (MRFIT) (n=361,662) Framingham Study (n=5209) CHD indications per 1000 40 (Deaths/1000) 10-year CHD death rate 50 30 20 10 150 125 100 75 50 25 0 0 150 200 250 300 Serum cholesterol (mg/dL) 1% reduction in total cholesterol resulted in a 2% decrease in CHD risk Gotto AM Jr, et al. Circulation. 1990;81:1721-1733. Castelli WP. Am J Med. 1984;76:4-12. 204 205-234 235-264 265-294 295 Serum cholesterol (mg/100 mL) Each 1% increase in total cholesterol level is associated with a 2% increase in CHD risk Relationship Between Cholesterol and CHD Risk: Epidemiologic Trials (cont’d) Okinawa, Japan 400 40 Men CHD mortality rates (%) per 100,000 screened subjects in 2 years Cumulative incidence of AMI 500 Seven Countries Study† Women 300 200 100 20 10 0 0 Range Mean 30 Northern Europe Southern Europe, Mediterranean United States Serbia Southern Europe, Inland Japan 167 149.3 168-191 179.8 192-217 203.7 218 245.3 Serum cholesterol (mg/dL) Cumulative incidence of acute myocardial infarction (AMI) increased with the level of serum cholesterol Cumulative incidence of AMI 125 175 225 275 Serum cholesterol (mg/dL) Using linear approximation, a 20-mg/dL increase in total cholesterol corresponded to a 17% increase in mortality risk in each quartile of basal serum cholesterol, expressed per 100,000 screened subjects in 3 years. Serum cholesterol was measured between April 1, 1983 and March 31, 1984. † 25-year CHD mortality rates per baseline cholesterol quartile adjusted for age, cigarette smoking, and systolic blood pressure. Wakugami K, Iscki R, Kimura Y, et al. Japanese Circulation Journal. 1998;62:7-14. Verschuren WMM, Jacobs DR, Bloemberg BPM, et al. JAMA. 1995;274:131-136. 325 Atorvastatin Calcium (LIPITOR®): Mechanism of Action HMG-CoA reductase inhibition Reduced cholesterol synthesis Upregulation of LDL receptors Decreased VLDL production Increased removal and catabolism of LDL Decreased TC, LDL-C, and TG Conversion of VLDL to LDL Atorvastatin Calcium (LIPITOR®) Indications An adjunct to diet to reduce elevated total-C, LDL-C, apoB, and TG levels in patients with primary hypercholesterolemia (heterozygous familial and nonfamilial) and mixed dyslipidemia (Fredrickson Types IIa and IIb) As adjunctive therapy to diet for the treatment of patients with elevated serum triglyceride levels (Fredrickson Type IV) For the treatment of patients with primary dysbetalipoproteinemia (Fredrickson Type III) who do not respond adequately to diet To reduce total-C and LDL-C in patients with homozygous familial hypercholesterolemia as an adjunct to other lipid-lowering treatments (eg, LDL apheresis) or if such treatments are unavailable The effect of LIPITOR on cardiovascular morbidity and mortality has not been determined Mean % LDL-C reduction from baseline Atorvastatin Calcium (LIPITOR®) Dose-Response Relationship 0% 10 mg 20 mg 40 mg 80 mg -10% -20% -30% -40% -41% -50% -60% -70% P<0.05 vs placebo. Nawrocki JW, et al. Arterioscler Thromb Vasc Biol. 1995;15:678-682. -44% -50% -61% In Head-to-Head Trials, Atorvastatin Calcium Showed Superior LDL-C Reductions at Starting Doses Mean % LDL-C change from baseline 0% -5% atorvastatin simvastatin (n=132) (n=45) 10 mg 10 mg atorvastatin (n=222) pravastatin (n=77) atorvastatin (n=707) lovastatin (n=191) 10 mg 20 mg 10 mg 20 mg -10% -15% -20% -23% -27% -25% -30% -30% -35% -37% -40% Dart et al. -35% P 0.05 Data on file. Parke-Davis; Morris Plains, NJ. Dart A, et al. Am J Cardiol. 1997;80:39-44. Bertolini S, et al. Atherosclerosis. 1997;130:191-197. Davidson M, et al. Am J Cardiol. 1997;79:1475-1481. Bertolini et al. -36% P 0.05 Davidson et al. P 0.05 The impact on clinical outcomes of the differences in lipid-altering effects between treatments shown in this slide is not known. This chart does not contain data comparing the effects of atorvastatin with higher doses of simvastatin, pravastatin, and lovastatin. Mean % triglyceride change from baseline In Head-to-Head Trials, Atorvastatin Calcium Showed Superior TG Reductions at Starting Doses 0% atorvastatin (n=132) 10 mg simvastatin (n=45) 10 mg atorvastatin (n=222) pravastatin (n=77) atorvastatin (n=707) lovastatin (n=191) 10 mg 20 mg 10 mg 20 mg -6% -5% -9% -10% -15% -15% -20% -25% -17% -17% -23% Dart et al. P 0.05 Data on file. Parke-Davis; Morris Plains, NJ. Dart A, et al. Am J Cardiol. 1997;80:39-44. Bertolini S, et al. Atherosclerosis. 1997;130:191-197. Davidson M, et al. Am J Cardiol. 1997;79:1475-1481. Bertolini et al. P 0.05 Davidson et al. P 0.05 The impact on clinical outcomes of the differences in lipid-altering effects between treatments shown in this slide is not known. This chart does not contain data comparing the effects of atorvastatin with higher doses of simvastatin, pravastatin, and lovastatin. CURVES Trial Design and Objective CURVES is a multicenter, open-label, randomized, parallelgroup, 8-week, comparative-dose efficacy and safety study of once-daily atorvastatin compared with simvastatin, pravastatin, lovastatin, and fluvastatin in patients with elevated LDL-C Designed to assess comparative efficacy and safety of atorvastatin relative to simvastatin, pravastatin, lovastatin, and fluvastatin on lipids in patients with baseline LDL-C 160 mg/dL and TG 400 mg/dL Jones P, Kafonek S, Laurora I, Hunninghake D, for the CURVES Investigators. Am J Cardiol. 1998;81:582-587. CURVES Trial: Mean Baseline LDL-C Levels Treatment arm (mg) Mean baseline LDL-C (mg/dL) No. of patients atorvastatin 10 20 40 80 213 213 206 213 n=73 n=51 n=61 n=10 simvastatin 10 20 40 207 230 219 n=70 n=49 n=61 pravastatin 10 20 40 225 237 215 n=14 n=41 n=25 lovastatin 20 40 80 244 218 219 n=16 n=16 n=11 fluvastatin 20 40 236 192 n=12 n=12 Treatment arm sizes were powered to show statistical significance vs mg-equivalent dose of the other agents. Jones P, Kafonek S, Laurora I, Hunninghake D, for the CURVES Investigators. Am J Cardiol. 1998;81:582-587. CURVES Trial: Patient Inclusion Criteria and Demographics Patient inclusion criteria At two consecutive visits — LDL-C 160 mg/dL — TG 400 mg/dL Men and women 18 and 80 years of age Body Mass Index (BMI) 32 kg/m2 Patient demographics Mean age: 55.2 years Modified intent-to-treat patients 59% male — 41% female — Family history of CHD: 37% Smokers: 13% Average BMI: 26.7 kg/m2 Evenly Jones P, Kafonek S, Laurora I, Hunninghake D, for the CURVES Investigators. Am J Cardiol. 1998;81:582-587. distributed across treatment groups. CURVES Trial: LDL-C Reductions vs Other Statins -10 -20 Mean % change in LDL-C -30 † † pravastatin ‡ † -40 simvastatin ‡ lovastatin ‡ -50 (40 mg bid) atorvastatin (80 mg qd) -60 10 20 40 Dose range (mg) 80 Significantly less than atorvastatin 10 mg (P<0.02). Significantly less than atorvastatin 20 mg (P<0.01). ‡ Significantly greater than mg-equivalent doses of comparative agents (P0.01). † The impact on clinical outcomes of the differences in lipid-altering effects between treatments shown in this chart is not known. Jones P, Kafonek S, Laurora I, Hunninghake D, for the CURVES Investigators. Am J Cardiol. 1998;81:582-587. CURVES Trial: Superior LDL-C Reductions at Usual Starting Doses atorvastatin simvastatin pravastatin lovastatin fluvastatin 10 mg 20 mg 20 mg 20 mg 0 10 mg Mean % LDL-C reduction -10 -17%† -20 -24%† -28%† -30 -40 † -38% -29%† atorvastatin 10 mg efficacy (38%) Most commonly prescribed doses for each product. Source: IMS National Prescription Audit; February 1998. Significantly less than atorvastatin 10 mg (P<0.01). The impact on clinical outcomes of the differences in lipid-altering effects between treatments shown in this chart is not known. Jones P, Kafonek S, Laurora I, Hunninghake D, for the CURVES Investigators. Am J Cardiol. 1998;81:582-587. CURVES Trial: Superior LDL-C Reductions at Usual First Titrations atorvastatin simvastatin pravastatin lovastatin fluvastatin 0 20 mg Mean % LDL-C reduction 20 mg 40 mg 40 mg 40 mg -10 -20 -23%† -30 -31%† -35%† -40 -50 † -34%† atorvastatin 10 mg efficacy (38%) -46% Most commonly prescribed doses for each product. Source: IMS National Prescription Audit; February 1998. Significantly less than atorvastatin 20 mg (P<0.01). The impact on clinical outcomes of the differences in lipid-altering effects between treatments shown in this chart is not known. Jones P, Kafonek S, Laurora I, Hunninghake D, for the CURVES Investigators. Am J Cardiol. 1998;81:582-587. CURVES Trial: LDL-C Reductions at Every Dose Mean % LDL-C reduction from baseline 0 -15 -20 -35 -40 20 mg 10 mg simvastatin 10 mg 20 mg 20 mg lovastatin fluvastatin -30 10 mg atorvastatin pravastatin -25 20 mg 20 mg -45 -50 40 mg 40 mg 40 mg 40 mg 80 mg (40 mg bid) 40 mg Mean LDL-C reductions at 80-mg doses: LIPITOR® 80 mg (qd)=54% vs lovastatin 80 mg (40 mg bid)=48%, P>0.05. The impact on clinical outcomes of the differences in lipid-altering effects between treatments shown in this chart is not known. Jones P, Kafonek S, Laurora I, Hunninghake D, for the CURVES Investigators. Am J Cardiol. 1998;81:582-587. -55 80 mg (qd) CURVES Trial: Conclusions At usual starting doses and usual first titrations, atorvastatin demonstrated significantly greater LDL-C reductions vs other statins All statins were well tolerated and no serious adverse events were considered related to any of the study medications Jones P, Kafonek S, Laurora I, Hunninghake D, for the CURVES Investigators. Am J Cardiol. 1998;81:582-587. Treat-to-Target Trial: Design and Objectives A 54-week, open-label, randomized study comparing the efficacy and safety of atorvastatin fluvastatin lovastatin simvastatin in the reduction of LDL-C levels to NCEP goals Data on file. Parke-Davis; Morris Plains, NJ. Treat-to-Target Trial: Patient Entry Criteria and Demographics Lipid entry criteria — LDL-C: patients with <2 risk factors (190 mg/dL), patients with 2 risk factors (160 mg/dL) — TG: all patients (400 mg/dL) LDL-C range: 129-448 mg/dL; LDL-C mean: 205 mg/dL Demographics — mean age: 56 years (53% women, 47% men) — 24% had <2 CHD risk factors, 76% had 2 risk factors — baseline LDL-C values were similar for all treatment groups Data on file. Parke-Davis; Morris Plains, NJ. Treat-to-Target Trial: Dosing Schedule Statin atorvastatin Dosing schedule 20 mg 40 mg fluvastatin 20 mg 40 mg lovastatin 20 mg 40 mg 20 mg 40 mg simvastatin 10 mg 10 mg Data on file. Parke-Davis; Morris Plains, NJ. With colestipol 80 mg 80 mg 5 g bid 5 g bid 10 g bid 5 g bid 10 g bid 5 g bid 10 g bid Treat-to-Target Trial: Study Design 8-week dietary assessment (optional) 4-week lead-in 344 patients randomized (1:1:1:1) atorvastatin vs fluvastatin vs lovastatin vs simvastatin for 54 weeks to reach <160 mg/dL for patients with <2 risk factors (24%) or <130 mg/dL for patients with 2 risk factors (76%) 12 weeks atorvastatin 10 mg (n=85) 12 weeks fluvastatin 20 mg (n=82) 12 weeks lovastatin 20 mg (n=83) If not at NCEP LDL-C goal, titrate at 12-week intervals for 54 weeks to maximum dose and add colestipol if needed Data on file. Parke-Davis; Morris Plains, NJ. 12 weeks simvastatin 10 mg (n=87) Treat-to-Target Trial: 12-Week Results 80 70 71% 60 % Patients reaching NCEP LDL-C goal by week 12 50 40 34% 30 16% 20 10 0 Significantly 41% 10 mg atorvastatin (n=85) 20 mg fluvastatin (n=82) 20 mg 10 mg lovastatin (n=83) simvastatin (n=87) less than atorvastatin 10 mg (P<0.001). The impact on clinical outcomes of the differences in lipid-altering effects between treatments shown in this chart is not known. Data on file. Parke-Davis; Morris Plains, NJ. Treat-to-Target Trial: 24-Week Results 100 90 80 70 % Patients 60 reaching NCEP 50 LDL-C goal by week 24 40 30 20 10 0 Significantly 84% atorvastatin 10 mg efficacy at week 12 46% 53% 28% atorvastatin 10-20 mg (n=85) fluvastatin 20-40 mg (n=82) lovastatin 20-40 mg (n=83) simvastatin 10-20 mg (n=87) less than atorvastatin (P<0.001). The impact on clinical outcomes of the differences in lipid-altering effects between treatments shown in this chart is not known. Data on file. Parke-Davis; Morris Plains, NJ. Treat-to-Target Trial: Summary of Results Compared with patients on fluvastatin, lovastatin, and simvastatin, significantly more patients on atorvastatin reached NCEP LDL-C goal at usual starting doses by every study interval with fewer titrations in fewer office visits 95% of those on atorvastatin reached LDL-C goal by end of study—all on monotherapy Data on file. Parke-Davis; Morris Plains, NJ. Effective for a Broad Range of Patient Populations LDL-C and TG reductions with atorvastatin at the 10-mg starting dose Elderly (>70 years) Women (n=183) Mean % change from baseline 0% LDL-C TG (n=689) LDL-C TG Hypertensives (n=510) LDL-C TG CHD (n=196) LDL-C TG NIDDM (n=156) LDL-C TG Mixed dyslipidemics (n=550) LDL-C TG -5% -10% -15% -18% -18% -21% -20% -21% -21% -24% -25% -30% -35% -39% -38% -37% -37% -36% -40% Pooled results from separate trials of atorvastatin 10 mg in more than 2000 patients for up to 52 weeks. Data on file. Parke-Davis; Morris Plains, NJ. -35% Atorvastatin Calcium in Hard-to-Treat Patient Populations Atorvastatin 80 mg in severe hypercholesterolemia (baseline total cholesterol 368 mg/dL; LDL-C 289 mg/dL) Mean % change from baseline 10% 0% +7% Total-C LDL-C TG ApoB HDL-C -10% -20% -33% -30% -40% -50% -60% Data on file. Parke-Davis; Morris Plains, NJ. -44% -46% -53% Economic Considerations in the Management of Hypercholesterolemia Hypercholesterolemia CHD events Adverse events Medications Physician visits Hospitalization Dietary counseling Monitoring Surgeries Titration Cost Considerations in LDL-C Reduction CURVES Trial Results: Monthly cost Percent LDL-C reduction 0 -5 -10 -15 -20 -25 -30 -35 -40 -45 -50 -55 -60 atorvastatin $54.72 $84.60 $101.88 simvastatin $62.99 $109.88 $109.88 10 mg 20 mg 40 mg -38% -46% -51% 10 mg 20 mg 40 mg -28% -35% -41% pravastatin $60.33 $64.95 $106.77 10 mg -19% 20 mg 40 mg -24% -34% lovastatin $69.85 $125.74 20 mg 40 mg -29% -31% fluvastatin $37.70 $37.70 20 mg 40 mg -17% -23% Jones P, Kafonek S, Laurora I, Hunninghake D, for the CURVES Investigators. Am J Cardiol. 1998;81:582-587. Red Book® Update. Montvale, NJ: Medical Economics Data Production Company. August 1998;46,47,51,59,71. LIPITOR® 10 mg (n=73), 20 mg (n=51), 40 mg (n=61), 80 mg (n=10); simvastatin 10 mg (n=70), 20 mg (n=49), 40 mg (n=61); pravastatin 10 mg (n=14), 20 mg (n=41), 40 mg (n=25); lovastatin 20 mg (n=16), 40 mg (n=16), 80 mg (n=11); fluvastatin 20 mg (n=12), 40 mg (n=12). Baseline LDL-C values were similar for all groups. LDL-C reductions at 80-mg doses: LIPITOR 80 mg (qd)=54% vs lovastatin 80 mg (40 mg bid)=48%, P>0.05. Average wholesale price (AWP) per tablet provided by Red Book® Update, August 1998. Actual pharmacy or out-of-pocket costs may differ. Cost comparisons do not imply comparable efficacy or safety. Dosage price for LIPITOR and lovastatin 80 mg is equal to double their 40-mg tablet cost. Atorvastatin Calcium: Great Value at Every Dose Dose AWP cost/day AWP cost/month atorvastatin 10 mg 20 mg 40 mg $1.82 $2.82 $3.40 $54.72 $84.60 $101.88 simvastatin 10 mg 20 mg 40 mg 80 mg $2.10 $3.66 $3.66 $3.66 $62.99 $109.88 $109.88 $109.88 pravastatin 10 mg 20 mg 40 mg $2.01 $2.17 $3.56 $60.33 $64.95 $106.77 lovastatin 20 mg 40 mg $2.33 $4.19 $69.85 $125.74 fluvastatin 20 mg 40 mg $1.26 $1.26 $37.70 $37.70 cerivastatin 0.2 mg 0.3 mg $1.32 $1.32 $39.60 $39.60 Red Book ® Update. Montvale, NJ: Medical Economics Data Production Company. August 1998;23,46,47,51,59,71. Atorvastatin Calcium Pharmacokinetics Absorption — rapidly absorbed — maximum plasma concentrations in 1 to 2 h — absorption increases in proportion to dose Bioavailability — absolute: ~14% — systemic: ~30% Distribution — mean Vd ~381 L — 98% bound to plasma proteins Atorvastatin Calcium Pharmacokinetics (cont’d) Metabolism — metabolized by hepatic cytochrome P450 3A4 — ~ 70% of inhibitory activity attributed to active metabolites Excretion — eliminated primarily in bile — mean plasma elimination half-life ~14 h Half-life for inhibitory effects is 20 to 30 h (due to contribution of active metabolites) Well Tolerated in Clinical Trials In head-to-head clinical trials, atorvastatin provided discontinuation rates comparable with other statins Discontinuation due to associated adverse events atorvastatin (n=1148) 2.7% Combined statins (n=383) 3.4% Pooled data from three comparative trials of patients taking either atorvastatin (10-20 mg), simvastatin (10-20 mg, n=45), pravastatin (20-40 mg, n=78), or lovastatin (20-40 mg, n=260) for 52 weeks. Atorvastatin Calcium Safety Profile In clinical trials, the most common adverse events were constipation, flatulence, dyspepsia, and abdominal pain It is recommended that liver function tests be performed prior to and at 12 weeks following both the initiation of therapy and any elevation of dose, and periodically thereafter LIPITOR is contraindicated in patients with hypersensitivity to any component of this medication; in patients with active liver disease or unexplained persistent elevations of serum transaminases; in women during pregnancy and in nursing mothers With any statin, tell patients to promptly report muscle pain, tenderness, or weakness. Discontinue drug if myopathy is suspected, if creatine phosphokinase (CPK) levels rise markedly, or if the patient has risk factors for rhabdomyolysis Atorvastatin Calcium Safety Profile (cont’d) Contraindications — hypersensitivity to atorvastatin components — active liver disease — unexplained persistent elevations of serum transaminases — pregnancy and lactation Potential drug interactions — cyclosporine — fibric acid derivatives — niacin — erythromycin — azole antifungals — digoxin — oral contraceptives Atorvastatin for a Broad Range of Hypercholesterolemic Patients 72% of patients reach NCEP LDL-C goal at the 10-mg starting dose Greater LDL-C reductions vs other statins at milligram-equivalent doses across the 10- to 40-mg dose range Reduces LDL-C 39% to 60% across the 10- to 80-mg dose range Reduces TG 19% to 37% across the 10- to 80-mg dose range Increases HDL-C 5% to 9% 10-mg starting dose lowers lipid measures (TC, TG, apoB) significantly more than simvastatin, lovastatin, or pravastatin in comparative trials Indicated to reduce elevated LDL-C and TG in patients with hypercholesterolemia A multicenter, double-blind study of all hypercholesterolemic patients taking LIPITOR (10 mg, n=707) for 16 weeks. Baseline lipid level:192 mg/dL. Target NCEP LDL-C goal based on CHD risk status, percentage of patients reaching goal, and total number of patients: <2 CHD risk factors, <160 mg/dL, 95%, n=329; 2 CHD risk factors, <130 mg/dL, 67%, n=268; with CHD, 100 mg/dL, 18%, n=110. Data on file. Parke-Davis; Morris Plains, NJ. Nawrocki JW, et al. Arterioscler Thromb Vasc Biol. 1995;15:678-682. Dart A, et al. Am J Cardiol. 1997;80:39-44. Bertolini S, et al. Atherosclerosis. 1997;130:191-197. Davidson M, et al. Am J Cardiol. 1997;79:1475-1481.