* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Clinical Pharmacology of Antiretroviral Therapy
Pharmacokinetics wikipedia , lookup
Psychopharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug interaction wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Prescription costs wikipedia , lookup
Theralizumab wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Pharmacogenomics wikipedia , lookup
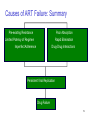
Changing Anti-Retroviral Therapy HIV Care and ART: A Course for Pharmacists Introductory Case: Mikael Mikael is a 28 year-old male diagnosed with AIDS who has been taking his triple drug ART regularly for the past 6 months without difficulties. He began therapy one year ago, during a bout of PCP pneumonia and oral thrush CD4/TLC and VL monitoring is not available in his region Today Mikael is diagnosed with toxoplasmosis and is hospitalized for treatment with Fansidar 2 Introductory Case: Mikael (cont.) Which of the following statements about changing therapy are true? 1. ART should not be changed because this patient is not experiencing side effects from his regimen 2. A change in ART should be done for a patient who experiences a new opportunistic infection on ART 3. ART should be changed as soon as a patient starts to miss doses to avoid treatment failure 4. ART should only be changed when a patient experiences virologic failure 3 Unit Learning Objectives Identify reasons for changing ART List factors in ART failure Determine how to change ART due to toxicity, treatment failure or concomitant disease Describe factors to consider when changing ART Describe appropriate laboratory monitoring procedures for ART 4 Factors to Consider When Changing Regimen Ethiopia ARV guidelines Prior antiretroviral history Antiretroviral resistance Side effects Number of drugs needing replacement Barriers to adherence Patient life-style and preferences Ability to follow-up in clinic Drug interactions Cost and sustainability 5 Reasons for Changing ART ART is not changed unless absolutely necessary! ART may be changed because of: Treatment Failure • Clinical failure • Immunologic failure • Virologic failure Toxicity or intolerance Co-morbid conditions Non-adherence/compromised quality of life 6 Treatment Failure Treatment failure is defined by Clinical failure • New or recurrent OI • Onset or recurrent WHO Stage III condition Note: Should not be confused with immune reconstitution inflammatory syndrome Immunologic failure • Fall of CD4 count by >50% from the peak • Return of the CD4 count to baseline or below 7 Introductory Case: Mikael (cont.) 1. ART should not be changed because this patient is not experiencing side effects from his regimen FALSE Where CD4 counts and viral load tests are unavailable, the WHO recommends using clinical evaluation to define treatment failure This patient is experiencing clinical failure defined as the development of a new opportunistic infection (in this case toxoplasmosis) while on ART 8 Introductory Case: Mikael (cont.) 2. A change in ART should be done for a patient who experiences a new opportunistic infection on ART TRUE Where CD4 counts and viral load tests are unavailable, the WHO recommends using clinical evaluation to define treatment failure 9 Treatment Failure (2) Virologic failure: Failure to suppress viral load to undetectable Reappearance of detectable virus after a period of undetectability (loss of virologic control) Less than one log (10-fold) decrease in viral load from baseline after 8-12 weeks of ART 10 Antiretroviral Therapy: Failure to Suppress 100000 Medications Started HIV RNA 10000 1000 100 50 10 50 TIME Courtesy of David H. Spach, MD; NW AETC, University of Washington 11 Antiretroviral Therapy: Viral Failure 100000 Medications Started HIV RNA 10000 1000 100 10 50 TIME 50 12 Courtesy of David H. Spach, MD; NW AETC, University of Washington Reasons Treatment May Fail Treatment fails if: Drugs are not strong enough to control the virus Patient is too sick (clinical failure) or has serious infections which are not treatable Patient has poor adherence • Missing more than three doses/month increases risk of treatment failure • Missing additional doses causes drug levels to fall, making HIV resistant 13 Treatment Failure and IRIS Must differentiate treatment failure from Immune Reconstitution Inflammatory Syndrome (IRIS) Clinical manifestation of a sub-clinical infection present at baseline. Brought on by ART-induced reconstitution of the immune system Typically seen within several weeks of initiating ART 14 Introductory Case: Mikael (cont.) 3. ART should be changed as soon as a patient starts to miss doses to avoid treatment failure FALSE If it is determined that a patient is missing doses, it is best to try to determine the cause and to identify a solution to assist the patient with adherence Changing ART should only be done when absolutely necessary 15 Reasons Treatment May Fail (2) Other medicines may stop ART from working Reduce levels of ARVs in the blood • e.g. TB drug rifampicin is a potent liver enzyme inducer Important: Patients must be warned that herbal medicines could reduce ARV drug levels Traditional healers must be told that their medicines could reduce ARV drug levels 16 Reasons Treatment May Fail (3) Patient cannot tolerate the available drugs Liver damage, nerve damage and anemia are possible serious side effects Diarrhea, nausea and vomiting caused by the drugs may sometimes be too much to bear Treatment may have to be stopped if these side effects become serious and replacement drugs are not available 17 Introductory Case: Mikael (cont.) 4. ART should only be changed when a patient experiences virologic failure FALSE Virologic failure is only one possible reason that a change in therapy may be necessary Other reasons to change therapy include: • Intolerable toxicities or side effects • Treatment failure (clinical failure or immunologic failure) • Co-morbidities 18 Causes of ART Failure: Summary Pre-existing Resistance Poor Absorption Limited Potency of Regimen Rapid Elimination Drug-Drug Interactions Imperfect Adherence Persistent Viral Replication Drug Failure 19 Toxicity About 50% of patients treated for three years with good viral suppression will require a change in therapy due to an adverse reaction to antiretroviral drugs Intolerable side effects Organ Dysfunction Interventions: If the offending drug can be identified, replace just that drug If the offending drug cannot be identified, replace entire regimen 20 Clinical Indications to Change ART Due to Toxicity Symptom Clinical Indication Nausea Severe discomfort or minimal intake for > 3 days Vomiting Severe vomiting of all foods/fluids in 24 hrs, orthostatic hypotension or need of IV fluids Diarrhea Bloody diarrhea, orthostatic hypotension or need of IV fluids Fever Headache Allergic Reaction Unexplained fever of > 39.6 C Severe or requires narcotics Generalized urticaria, angioedema or anaphylaxis Peripheral Severe discomfort, objective weakness, loss of 2-3 Neuropathy previously present reflexes or sensory dermatomes Fatigue Normal activity reduced > 50% 21 Lab Indications to Change ART Due to Toxicity Parameter Grade 3 Toxicity < 750/mm3 M: 13.8 – 17.2 g/dL F: 12 – 15.6 g/dL 1500 to 7000/mm3 Platelet count < 49 x 103/µL 130-400 x 103/µL Total Bilirubin > 3-7.5 x ULN*= 3.9-9.75mg/dL ≤ 1.3 mg/dL SCr > 1.7-2.0 (adult) ≤ 1.2 mg/dL Hemoglobin (Hgb) < 7.0 g/dL Hematology ANC Chemistries AST / ALT LFTs Pancreatic Enzymes Normal Reference Values 5-10 x ULN* = ≤ 42 U/L , ≤ 48U/L 210-420 U/L, 240480 U/L Amylase, Lipase > 2-3 x ULN* 23-85 U/L, 0-160 U/L Triglyceride (TG) 8.49- 13.56 mmol/L < 200 mg/dL 1.6-2.0 X ULN < 200 mg/dL Lipids Cholesterol * ULN = Upper Limit of Normal 22 Toxicity: Changing One Drug Regimen: d4T/3TC/NVP d4T-related neuropathy or pancreatitis: Switch d4T to ZDV NVP-related rash or hepatotoxicity: • Switch NVP to EFZ (except pregnancy) • Switch NVP to PI’s (in cases of pregnancy or severe adverse effect) Regimen: d4T/3TC/EFV EFZ-related persistent CNS toxicity: Switch EFZ to NVP Regimen: ZDV/3TC/EFV ZDV-related anemia or neutropenia: Switch ZDV to d4T 23 Co-morbidities A change in clinical status of patients may mandate change in ART Pregnancy: • If on EFV based regimen – change EFV to NVP Occurrence of active TB: • If on NVP based regimen – change to EFV (with adjusted dose), to LPV/r, or SQV/r 24 Minimizing Viral Resistance Never prescribe ARVs in the absence of adherence counseling and support Work with patients and their families to minimize barriers to medication adherence Never prescribe ARV monotherapy or dual therapy If ARV medications are to be discontinued, stop all drugs at the same time 25 Minimizing Viral Resistance (2) Do not prescribe ZDV (zidovudine) and d4T (stavudine) together (antagonistic) Pay meticulous attention to other medications and treatments and their potential to interact with ARV therapies Never add a single drug (alone) to a failing regimen 26 Improving Adherence and Enhancing QOL Reduce pill burden Changing to fixed dose combinations Replace PIs with NNRTI’s or abacavir Minimize food/water restrictions Revisit co-morbid conditions that might be interfering, e.g. mental health; substance abuse Inquire about side effects that may have contributed to poor adherence 27 Changing Regimen When changing regimen due to treatment failure: Evaluate for resistance through resistance testing or empiric decision-making based on clinical history Change to an entirely new regimen, with at least one drug from a new class Anticipate some cross-resistance (e.g. ZDV and d4T) Try to determine and correct reasons for failure of the first regimen (e.g. adherence issues) 28 Alternative Regimen First Line Regimen 2nd Line Regimen for Tx Failure D4T or ZDV + 3TC + NVP or EFV ABC or TDF or ZDV (if not taken) + DDI + LPV/r or SQV/r OR NFV or IDV/r Source: Guideline for Use of Antiretroviral Drugs in Ethiopia. MOH, January 2005. p. 16 29 Changing Regimen (2) Second-line therapy for patients with drug failure on d4T/3TC/nevirapine or efavirenz: • ZDV + DDI + IDV/r or • TDF +DDI + LPV/r Second-line therapy for patients with drug failure on ZDV/3TC/nevirapine or efavirenz: • TDF + DDI + IDV/r or • TDF +DDI + LPV/r 30 Monitoring Therapy First-Line Regimen: Laboratory Monitoring Baseline: ALT and CD4 or TLC Additional lab monitoring varies with regimen NVP: ALT/AST at 2, 4, 6, 8 weeks, 3 months, then q 6 months and symptom directed EFV: symptom directed thereafter for ALT, pregnancy test at baseline for women of childbearing age ZDV: CBC/diff at baseline, 2, 4, 8 and 12 wks, then q 3-6 months and symptom directed 32 Laboratory Monitoring Guideline Regimen ART Lab Test 1 D4T/3TC/NVP ALT Other Frequency TLC or CD4 Baseline, 2, 4, & 8 wks, then q 6 months & symptom directed for toxicity Baseline & q 6 months D4T/3TC/EFV ALT TLC or CD4 Symptom directed Baseline & q 3-6 months ZDV/3TC/EFV ALT TLC or CD4 CBC + diff/ Hgb/Pltc Symptom directed Baseline & q 3-6 months Baseline, 4, and 12 wks, & thereafter symptom directed ZDV/3TC/NVP ALT TLC or CD4 CBC + diff/ Hgb/Pltc Refer to slide at end of handbook. Baseline, 2, 4, & 8 wks, then q 6 months then q 6 months & symptom directed for toxicity Baseline & q 3-6 months Baseline, 4, and 12 wks, & thereafter symptom directed Source: Guideline for Use of Antiretroviral Drugs in Ethiopia. MOH, January 2005. Second-Line Regimen: Laboratory Monitoring Baseline: CBC/diff, ALT, SCr and CD4 or TLC Other lab monitoring varies with regimen ZDV: CBC/diff at baseline, 2, 4, 8, 12 wks, then q 3-6 months and symptom directed DDI: amylase at baseline and symptoms of abdominal pain, CBC with diff and LFTs q 12 months TDF: urine protein dipstick and SCr at baseline, 3 months and q 6 months, CBC/diff and AST/ALT q 12 months PIs: lipids at baseline and q 12 months, AST/ALT at baseline, 3 months then q 6 months, fasting glucose at baseline, then q 12 months Indinavir: urine dipstick for RBCs with symptoms of flank pain, SCr at baseline then q 6 months 34 Case Studies Case 1 Case Study: Kasahun Kasahun is a 25 year-old male (CD4 count 56) who presents to clinic for follow-up 2 weeks after starting ZDV, 3TC and NVP. He learned his HIV status two months ago, during a hospitalization with CNS toxoplasmosis. He is currently receiving treatment for toxoplasmosis and has tolerated his medication over the past two months At diagnosis he had significant lower leg numbness and weakness due to INH therapy for TB Pyridoxine 40 mg was started to replace his B-complex vitamin. Why would this be necessary? 37 Case Study: Kasahun (2) He had some nausea the first week on meds, which he has resolved by eating small meals before doses. He claims to take his medications every day as directed. He has lost weight and is now 51 kg Today he presents complaining of a mildly itchy red rash over his trunk and arms which began 2 days ago. He says he feels tired all day. He proudly states that he has taken his NVP twice daily since his first day on medications 38 Case Study: Kasahun (3) 1. 2. 3. 4. What do you think is occurring with Kasahun? Should his ART regimen be changed? What additional information would you like to know? What are the laboratory tests that should be done at today’s visit to monitor Kasahun’s therapy? 39 Case Study: Kasahun Follow-up (4) Laboratory Values WBC: 5.6 H/H: 6.9 / 27 LFTs: 31 / 26 How would you interpret these lab results? He has had only minor headaches over the past 3 weeks. What does this tell us? He does not have any other areas of the rash, oral blisters, myalgias or fever. What should you do? Did he take NVP QD x 2 weeks, then increase to BID? 40 Case Study: Kasahun (5) He has not been having trouble remembering doses He has been taking doses with meals (breakfast and dinner) Peripheral neuropathy symptoms have improved However, he has been missing work due to fatigue 41 Case Study: Kasahun (6) 1. How would you interpret his results? 2. Would you change his ART? 3. How would you counsel Kasahun? 42 Case Study: Kasahun (7) Follow up at 3 months shows: CD4 count = 110 (10%) cell/mm3 Hemoglobin/Hematocrat = 13 / 38% WBC = 5.8 55 kg He is tolerating medications except for occasional numbness in lower extremities. His symptoms have somewhat improved over last 3 months. Occasional nausea after taking meds Claims he is taking all his medications. He takes his morning dose at work during his break and takes his evening dose with dinner 43 Case Study: Kasahun (8) Therapeutic goals are being met Patient energy increased and he’s feeling better Appetite is good and he has gained 4 Kg CD4 increasing (was 56/5% 2 months ago) H/H normalized However, he reports side effects: If possible, decrease dose of stavudine to 30mg BID for neuropathy Encourage food before doses 44 Case 2 Case Study: Yared Yared is a 30 year-old man who has been stable on stavudine, lamivudine and nevirapine for the past four years History: PCP pneumonia 4 years ago Today his CD4 cell count is 140 cells/mm3, his previous CD4 count was 300 and his viral load has risen from undetectable levels to 50,000 copies/ml He feels well and has no complaints today 46 Case Study: Yared (2) 1. 2. 3. 4. What do you think is happening to Yared? What additional information would you like to know? Does he require a change in his regimen? What possible regimen can you give to Yared, based on your local situation? 47 Case Study: Yared Follow-up (3) Yared had been taking his medication as prescribed but missed doses recently while he was visiting his brother in Jimma What are the factors to consider before starting a new regimen? 48 Case Study: Yared (4) Side effects Educate Yared on the potential side effects of each potential regimen Patient preference Yared is very fearful of the ABC hypersensitivity syndrome and would prefer to avoid ABC in his next regimen Review drug-drug interactions Cost and sustainability Barriers to adherence Plan ahead for changes in schedule, vacations, etc 49 Case Study: Yared (5) He will begin TDF 300 mg qd +DDI 250 mg qd +LPV/r 3 caps bid How will you monitor his therapy? Clinical Laboratory Does he have to take his DDI apart from LPV/r and/or tenofovir? 50 Key Points Treatment failure occurs because of preexisting resistance, limited regimen potency, imperfect adherence, poor absorption, rapid elimination, or drug-drug interactions Therapy should not be changed unless absolutely necessary The main reasons for changing ART are treatment failure and drug toxicity 51 Key Points (2) Other reasons for changing ART include problems with adherence or other medical conditions or illnesses Ongoing laboratory monitoring is necessary to detect all side effects and to monitor success/failure of therapy 52