* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Varicella
Behçet's disease wikipedia , lookup
Germ theory of disease wikipedia , lookup
Gastroenteritis wikipedia , lookup
Kawasaki disease wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Immunocontraception wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Herd immunity wikipedia , lookup
Neuromyelitis optica wikipedia , lookup
Poliomyelitis eradication wikipedia , lookup
Herpes simplex wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Marburg virus disease wikipedia , lookup
Whooping cough wikipedia , lookup
Meningococcal disease wikipedia , lookup
Infection control wikipedia , lookup
Globalization and disease wikipedia , lookup
Vaccination policy wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
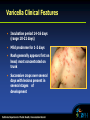
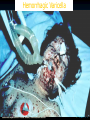
Varicella (Chickenpox) and Herpes Zoster (Shingles) Jennifer Zipprich Immunization Branch California Department of Public Health October 17th, 2012 Varicella-Zoster Virus (VZV) • Human alpha-herpesvirus • Causes varicella (chickenpox) and herpes zoster (shingles) • Primary VZV infection leads to chicken pox • VZV establishes latency in dorsal root ganglia after primary infection • VZV can reactivate at a later time, causing herpes zoster • There are 3 licensed vaccines to prevent varicella (Varivax®, Proquad®) and herpes zoster (Zostavax®) in the US: Varivax® (licensed 1995) Proquad® (licensed 2005) Zostavax® (licensed 2006) Varicella Clinical Features • Incubation period 14-16 days (range 10-21 days) • Mild prodrome for 1-2 days • Rash generally appears first on head; most concentrated on trunk • Successive crops over several days with lesions present in several stages of development Breakthrough Varicella • Breakthrough varicella is defined as infection with wild-type varicella disease occurring > 42 days after vaccination • Approximately 15-20% of 1-dose vaccinated persons may develop varicella if exposed to VZV • Usually milder clinical presentation than varicella in unvaccinated cases Usually low or no fever Develop < 50 lesions Experience shorter duration of illness Rash predominantly maculopapular rather than vesicular • 25-30% of breakthrough varicella cases are not mild and have clinical features more similar to varicella in unvaccinated persons Chaves J Infect Dis 2008; Arvin Clin Microb Rev 1996; CDC. Prevention of Varicella. MMWR 2007; 56(No. RR-4) Varicella: Complications • Secondary bacterial infection of skin lesions • Central nervous system manifestations (meningoencephalitis, cerebelllar ataxia) • Pneumonia (viral or bacterial) • Hepatitis, hemorrhagic complications, thrombocytopenia, nephritis occur less frequently • Certain groups at increased risk for complications Adults Immunocompromised persons Pregnant Women Newborns CDC. Prevention of Varicella. MMWR 2007; 56(No. RR-4); Arvin Clin Microb Rev 19 Hemorrhagic Varicella Varicella: Transmission • Transmitted person to person by direct contact, inhalation of aerosols from vesicular fluid of skin lesions of acute varicella or zoster, or aerosolized respiratory tract secretions • Period of contagiousness: 1-2 days before rash onset until all lesions crusted or disappear if maculopapular rash (typically 4-7 days) • Varicella in unvaccinated persons is highly contagious (61-100% secondary household attack rate) • Varicella in 1 dose-vaccinated persons half as contagious as unvaccinated cases CDC. Prevention of Varicella. MMWR 2007; 56(No. RR-4); Arvin Clin Microb Rev 1996; Seward JAMA 2004; Vaccines, 5th edition Herpes Zoster (Shingles) •Following initial infection (varicella), VZV establishes permanent latent infection in dorsal root and cranial nerve ganglia •Years to decades later VZV reactivates and spreads to skin through peripheral nerves causing pain and a unilateral vesicular rash in a dermatomal distribution •~1 million cases in the U.S. annually Clinical Features of Herpes Zoster Prodrome: headache, photophobia, malaise, fever, abnormal skin sensations and pain Rash: • Unilateral, involving 1-3 adjacent dermatomes • Thoracic , cervical, ophthalmic involvement most common • Initially erythematous, maculopapular • Vesicles form over several days, then crust over • Full resolution in 2-4 weeks • Occasionally, rash never develops (zoster sine herpete) Complications of Herpes Zoster • Postherpetic Neuralgia (PHN) Pain ≥ 30 days occurs in 18-30% of zoster cases Mild to excruciating pain after resolution of rash Constant, intermittent, or triggered by trivial stimuli May persist weeks, months or occasionally years Can disrupt sleep, mood, work, and activities of daily living and lead to social withdrawal and depression Risk factors for PHN include age ≥ 50, severe pain before or after onset of rash, extensive rash, and trigeminal or ophthalmic distribution of rash VZV Transmission from Zoster • VZV can be transmitted from persons with zoster • Risk of VZV transmission from zoster is much lower than from varicella • Transmission is mainly through direct contact with zoster lesions, although airborne transmission has been reported in healthcare settings • Localized zoster is only contagious after the rash erupts and until the lesions crust • Transmission from localized zoster can be decreased by covering the lesions Epidemiology Varicella Disease Burden in the United States Before Introduction of Varicella Vaccine in 1995 • • • • 4 million cases/year 11,0000 - 13,500 hospitalizations/year 100 - 150 deaths/year Greatest disease burden in children >90% cases 70% hospitalizations 50% deaths Wharton Infect Dis Clin North Am 1996; Galil Pediatr Infect Dis J 2002; Davis Pediatrics 2004; Meyer J Infect Dis 2000; Nguyen NEMJ 2005 Varicella Immunization • Varivax licensed in 1995 • In 1995 American Academy of Pediatrics recommended one dose of varicella vaccine for all children < 13, and for susceptible adolescents from 13-18 • In 1996 ACIP recommended vaccination for all children < 13 years of age; for susceptible adolescents and adults vaccination recommended for those at high risk of infection or complications. Vaccination of this group deemed desirable. Pediatrics 1995;95;791. Committee on Infectious Diseases; ACIP. Prevention of Varicella. 1996. Varicella Immunization • One dose program estimated to save $5 for every $1 spent on vaccine when factoring in parental time lost from work and direct medical costs When medical costs were considered alone each chicken pox case prevented would cost $2 • New Zealand – Total cost savings of $47 per child primarily driven by work-loss time averted • Germany, Taiwan, Singapore Varicella Immunization • Cost-Benefit typically analyze one dose programs • Use of MMRV often not considered • Costs related to hypothetical increase in zoster cases or increase in adult chicken pox cases not considered • High risk groups a better target? • Number of concerns raised include: waning immunity, potentially large pool of susceptible adults, serious complications rare Newman. Arch Pediatr Adolesc Med 1998; Lieu. JAMA 1994; Ross. BMJ. 1995. Varicella Active Surveillance Project (VASP) • VASP is a CDC-funded project initiated in 1995 in Philadelphia and Los Angeles County • Purpose of the active surveillance program To obtain population-based incidence rates for varicella and herpes zoster diseases in a community with established high varicella vaccination coverage rates to evaluate the impact of current and future varicella vaccination practices and policies Varicella Cases and 1-Dose Vaccine Coverage Varicella Active Surveillance Project Sites, 1995-2005 Varicella Cases 3500 100 3000 80 2500 1400 100 1200 80 1000 2000 60 1500 40 60 800 40 1000 600 20 400 20 500 0 200 0 0 1995 1997 1999 2001 2003 2005 Year Vaccination coverage 0 -20 1995 1997 1999 2001 2003 2005 Year Varicella cases 90% decline in varicella incidence in both sites Guris J Infect Dis 2008 Vaccine Coverage West Philadelphia Antelope Valley, California Number of Varicella Outbreaks Antelope Valley, CA 1995-2005 No. of Outbreaks 100 80 81 60 68 67 54 40 34 20 30 14 0 1995 1996 1997 1998 1999 2000 Year 2001 25 8 6 2002 2003 8 2004 2005 Number of Varicella Cases Antelope Valley, CA 1995-2005 Non-outbreak Cases Outbreak Cases 3500 No. of Cases 3000 2500 2000 1500 1000 500 0 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 Year Length of Outbreaks (Days) and Age of Cases (Years) Median Length Median Age 1995-1998 2002-2005 44.5 Days 30 Days 6 Years 9 Years Outbreak Cases: History of Disease or Vaccination and Disease Severity 1995-1998 2002-2005 History of prior varicella disease 6.3% 14% Breakthrough cases 1.6% 58% <50 Lesions 35% 45.7% Complicated disease 9.3% 3.6% Varicella and Measles Vaccine Coverage (1+ doses)*, Children 19-35 Months National Immunization Survey, 1997-2008 100 90 81 Coverage (%) 80 85 88 88 89 90 91 76 68 70 58 60 50 43 Varicella Measles 40 30 26 20 10 0 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 Year *National Immunization Survey (NIS), coverage available at http://www.cdc.gov/vaccines/stats-surv/default.htm#nis Varicella-Related Hospitalization Rates U.S., 1994-2002 Chart description of Varicella-Related Hospitalization Rates U.S. for 1994-2002. Rate per 100,000 Population. Prev-accination years of 1994, and 1995. Decline 1994-95 to 2002. Overall =88%. <10 yrs = 91%. 10-19 yrs=92%.20-49 yrs= 78%. Decline 1994-95 to 2002 Overall 88% < 10 yrs 91% 10-19 yrs 92% 20-49 yrs 78% Zhou et al, JAMA, 2005 Decline in Reported Varicella Deaths <50 years of age, US, 1990-2006 No. of Deaths average=85 93% decline in deaths in 2005-2006 compared to pre-vaccine era 1990-1994 average=8 YEAR National Center for Health Statistics Experience with 1-dose Varicella Vaccination Program • 1-dose varicella vaccination coverage in 19-35 month-olds increased from 26% to 91% from 1997 to 2008 • Varicella disease incidence declined by 90% in two varicella active surveillance sites by 2005 as compared to 1995 • Varicella hospitalizations declined 88% during 1994-2002 • Varicella mortality rate declined 93% from 1990-1994 to 2005-2006 in persons aged <50 years National Immunization Survey (www.cdc.gov/vaccines/stat-surv/default.htm#nis ); Guris J Infect Dis 2008; Marin Pediatrics 2008; Zhou JAMA 2005; National Center for Health Statistics Post-licensure One-Dose Vaccine Effectiveness in US* • 17 studies with 20 estimates Study designs: case-control, cohort (outbreaks, other), household contact • Prevention all varicella Median 85% (range 44% - 100%) Mean 81% • Prevention of combined moderate and severe varicella Median 97% (range 86% - 100%) Mean 96% • Prevention of severe varicella* Median 100% (range 97% to 100%) Mean 99% VARIVAX® Merck and Co. Inc; Seward J Infect Dis 2008 Impressive Achievements with the 1-Dose Varicella Vaccination Program But Challenges to Varicella Control Remained… • 15-20% of children vaccinated with 1 dose remain at risk for varicella due to lack of immune response or partial protection • Rationale for Timing of 2nd Dose of Varicella Vaccination at 4-6 Years of Age Low incidence among 1-4 year old children Outbreaks in elementary and middle schools Similar immune response to 2nd dose with intervals 3 months or 3-4 years after 1st dose Programmatic harmonization with MMR vaccine and availability of MMRV vaccine Current Varicella Vaccination Policy in the United States Implemented routine 2-dose childhood varicella vaccination program in 2006 1st dose at age 12-15 months 2nd dose at age 4-6 years Effectiveness is 98% for prevention of any primary varicella and 100% at prevention of severe disease CDC. Prevention of Varicella. MMWR 2007; 56(No. RR-4) Risk Factors for Herpes Zoster • Increasing age • Immunosuppression Bone marrow and solid organ transplantation Patients with hematological malignancies and solid tumors HIV Immunosuppressive medications • Gender: Increased risk in females • Race: Risk in African-American less than half that in Caucasians • Trauma or surgery in affected dermatome • Early varicella (in utero, infancy): Increased risk of pediatric zoster Age-specific Incidence of Herpes Zoster and Postherpetic Neuralgia: U.K., 1947-1972 Hope-Simpson J R Coll Gen Pract 1975. Varicella in California Immunization Branch at CDPH • • • • Surveillance and disease reporting to CDC Technical assistance to local health jurisdictions Educational materials Laboratory testing (VRDL) Varicella Reporting in California • • • • Outbreaks Hospitalizations Deaths HZ is not reportable Varicella Outbreak Management • • • • >=5 cases associated in time and place Exclusion of cases while infectious Provide immunization to susceptible contacts Provide VarzIG to high risk exposed susceptible contacts (pregnant women) • Exclude susceptible exposed children in a school setting? Reported School Outbreaks in California • Passively reported 2009: 31 outbreaks; Range 5 – 25 cases, median 7 cases 2010: 17 outbreaks; range 5 – 55 cases, median 7 cases 2011: 8 outbreaks; range 5 – 25 cases, median 7 cases • Many requests for technical assistance on outbreak management are related to exclusion of exposed unvaccinated children from school School Varicella Outbreak – October 2011 • Child in large unvaccinated family became infected with varicella (source unknown) • All children in family and pregnant mother became infected with varicella over a five week period; mother quite ill • Several siblings attend the same school and were the source of a school outbreak School Varicella Outbreak • School is K-8 with 208 students • 66 (38%) students have PBEs • 25 cases of varicella • 17 (68%) of the cases have PBEs • 2 cases had one dose of vaccine and one case had two doses • 1 pregnant teacher exposed California Law Granting Exclusion • The California Health and Safety Code section 120365 states “…whenever there is good cause to believe that the person [with a personal belief exemption] has been exposed to one of the communicable diseases listed in subdivision (a) of Section 120325, that person may be temporarily excluded from the school or institution until the local health officer is satisfied that the person is no longer at risk of developing the disease.” Pros of School Exclusion • Theoretically may slow a varicella outbreak • May reduce the number of infections and complications • May decrease likelihood of varicella to spread to high-risk people • May encourage parents to vaccinate children who would not otherwise be vaccinated • “Proactive” Cons of School Exclusion • No data that exclusion is effective in slowing a varicella outbreak • Immediate readmittance after vaccination may appear coercive • Childcare costs for parents of excluded children could be substantial • Long exclusion; children may suffer educationally • School law affects cohorts of children differently • Schools need to provide home education or risk losing attendance-based educational funds Outbreak of rash illness in a skilled nursing facility • • • • • • 3 Residents 4 employees 1 visitor (husband of a resident) Onset dates from 6/3/2012 – 6/21/2012 Ages ranged from 27 – 96 years VZV source was suspected to be resident with herpes zoster Age Clinical Affiliation Result Varicella Immune Status 40 lesions in various stages of development covering entire body and inside mouth Employee VZV detected IgG+ in 2008 6/19 65 Lesions; On chronic prednisone therapy Husband of resident VZV detected Presumed immune based on age 6/19 40 Minimal lesions, all dry Employee VZV detected History of disease 87 Typical lesions including vesicles Onset 6/18 6/19 Resident VZV detected Presumed immune based on age Similar report in the literature • Varicella transmission from HZ patient to 3 persons presumed immune in a long term care facility • Secondary cases were clinically compatible with chicken pox – though mild, <100 lesions and confirmed by PCR • Newly characterized varicella virus Varicella Reinfection? • Reinfection has been previously described but is rare Hall (2002) reviewed 9,947 varicella cases and found that between 4.5% and 13.3% of cases reported a history of varicella infection Case report on physician with prior evidence of serologic immunity Varicella Death, 2010 • 41 yo male previously healthy • Presented with 5 days history of abdominal pain and fever of 100.5 • Presented to the ER the following day after developing a generalized rash; discharged with acyclovir • Developed mild delirium and difficulty breathing • Complications included encephalitis and hepatitis • Patient expired 9 days after admission Varicella Death, 2007 • 13 month old previously healthy unvaccinated female • Presented to ED with fever to 102 and vesicular rash; diagnosed with chicken pox • Five days after rash onset patient became weak and unable to ambulate • Admitted and administered IV acyclovir • Patient expired 6 days after rash onset Questions? Acknowledgements • Centers for Disease Control and Prevention • Kathy Harriman • Teresa Lee • CDPH Immunization Branch California School Immunization Law • Immunization requirements at kindergarten entry: 4+ DTaP, 3+ polio, 2 MMR, 3 hepatitis B, 1 varicella • Exemptions and procedure Permanent and Temporary Medical Personal Beliefs (PBE) “shall be granted upon filing with the [school] a letter or affidavit from the pupil’s parent…that such immunization is contrary to his or her beliefs” PBE Study – California, 2009 • Two-fold Purpose To evaluate vaccination status of kindergarten PBEs Determine whether ‘high’ PBE schools were different from ‘standard’ PBE schools • Method Collected and analyzed PBE records from: a random sample of kindergartens and the top 50 PBE kindergartens Percent of Nonblank PBE Records Missing All Doses in Series 50 % of PBEs 40 28.9 30 20 10 41.8 Random Top 50 16.1 4 3.7 30.2 33.3 17.5 13.5 7.1 0 DTaP Polio Hep B MMR Var Increase in Permanent Medical (PME) and Personal Beliefs (PBE) Exemptions Among Kindergarten Students, California 1977-2009 Percent of Students 2.5% 2.0% Measles, mumps and rubella requirement Measles outbreak Varicella requirement Hepatitis B requirement 1.5% 1.0% 0.5% 0.0% 1977 1979 1981 1983 1985 1987 1989 1991 1993 1995 1997 1999 2001 2003 2005 2007 2009 PME PBE Year of Assessment