* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download NOTES: Carbohydrates / Lipids
Survey
Document related concepts
Transcript
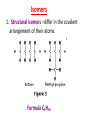
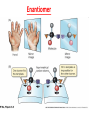
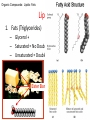
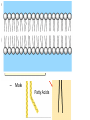
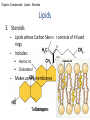
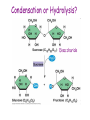
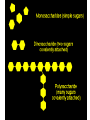
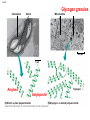
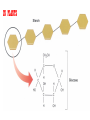
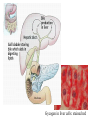
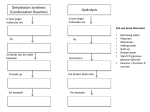
GFP gene = Green Fluorescent Protein RFP gene = Red Fluorescent Protein Let’s Back Up Let’s talk about the architecture of organic molecules…… • Isomer = the same molecular formula but different structures SO different properties. Isomers 1. Structural isomers –differ in the covalent arrangement of their atoms. Formula C4H10 Isomers 2. Geometric Isomers –differ in their spatial arrangement but have the SAME covalent bonds. Double bond makes them more inflexible-cannot rotate freely like in single bond! (variation in arrangement around a double bond) Isomers 3. Enantiomers –variation in spatial arrangement around asymmetric carbon. Result: molecules that are mirror images of each other (Left and Right Handed). Usually one is active and the other inactive in the body. (arrangement of the four spots around asymmetric carbon) Important for pharmaceutical companies? Enantiomer Isomers Enantiomers –Important for pharmaceutical companies? Why? Example: 1960- Thalidomide-ease pregnancy discomfort • Drug mixture of 2 enantiomers • 1 enantiomers-sedative • Other- side effects – birth defects LIPIDS • • • • • diverse group of organic compounds: grouped together because HYDROPHOBIC insoluble in water will dissolve in nonpolar solvents not a true polymer; still a macromolecule (C and H) include: 1. Fats 2. Phospholipids 3. Steroids Organic Compounds: Lipids: Fats Lipids 1. Fats (Triglycerides) – Glycerol + 3 Fatty Acids – Saturated = No Double Bonds (solid) – Unsaturated = Double Bonds (liquid) OH OH OH OH OH Ester Bonds OH 1. FATS • Composed of: – glycerol (3-carbon alcohol; each with a Hydroxyl group) – fatty acid (contains carboxyl group; long hydrocarbon chain or “tail”) • the nonpolar C-H bonds make the chain hydrophobic and insoluble in water • Fatty acids may vary in # of carbon atoms (usually even #) • Each of glycerol’s 3 hydroxyl groups can bond to a fatty acid by an ester linkage producing a fat. (resulting in triacylglycerol, or a triglyceride) Organic Compounds: Lipids: Phospholipids Lipids 2. Phospholipids – Glycerol with Phosphate Head + 2 Fatty Acid Chains – Amphiphilic (“Both” “lover”) • • Hydrophilic head Phosphate Hydrophobic tail Glycerol – Forms 2 layers in water – Makes up cell membranes Fatty Acids • clusters in water in patterns (e.g. micelle, bilayer) • shows ambivalent behavior towards water (tails are hydrophobic and heads are hydrophilic) Organic Compounds: Lipids: Steroids Lipids 3. Steroids – – Lipids whose Carbon Skeleton consists of 4 fused OH rings O OH O Includes: • • – HO Hormones Cholesterol Makes up cell membranes HO O Testosterone Estrogen OH Biotechnology • To extract DNA and other organelles from a cell, the phospholipid bilayer must be dissolved. This is achieved by the use of detergents which disrupts the hydrocarbon tails. I. Monosaccharides = single/simple sugars • major nutrients for cells • glucose is most common (C6H12O6) • store energy in their chemical bonds which is harvested by cellular respiration *examples: glucose, ribose, galactose Classification of Monosaccharides Catorized depending on: 1. Location of carbonyl group either Aldose (Aldehyde sugar) or Ketose (Ketone sugar) 2. Size of carbon skeleton ~3-7 carbons long (most form rings in solution) 3. Isomers – spatial arrangement around asymmetrical carbon ***Shape = function /interaction in body! Classification of Monosaccharides Aldose Structural Isomer of glucose=Ketose II. Polysaccharides = hundreds or thousands of monosaccharides • formed by linking monomers dehydration synthesis (condensation) reactions • Monomers held together by covalent bonds called glycosidic linkages Condensation or Hydrolysis? Glycosidic Linkage Condensation or Hydrolysis? Disaccharide A. Examples of energy storage polysaccharides: • Starch = glucose polymers in plants (amylose/amylopectin) • Glycogen = glucose polymer in animals LE 5-6 Glycogen granules Chloroplast Starch Mitochondria 0.5 µm 1 µm Amylose Glycogen Amylopectin Starch: a plant polysaccharide Glycogen: an animal polysaccharide IN PLANTS Digestion = Hydrolysis Difference in most carbohydrates is how the glucose monomers are connected! Gycogen in liver cells: stained red B. Examples of structural support polysaccharides: • cellulose = major structural component of plant cell walls that cannot be digested by most organisms because of missing digestive enzyme • chitin = forms exoskeletons of arthropods B. Examples of structural support polysaccharides: • chitin = Know the difference between the 5-carbon sugars!!! Biotechnology • Use glucose as food source for cell cultures. Biotechnology • Carbs also affect DNA purifications in plant cells because they are too sticky