* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch 3 Matter & Change
Survey
Document related concepts
Transcript
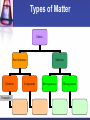
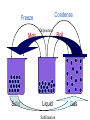
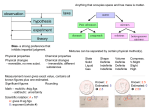
Matter – Properties and Changes What is Matter? Matter is anything that takes up space and has mass. Mass is the amount of matter in an object. Types of Matter Matter Pure Substance Elements Examples: Compounds Mixtures Heterogeneous Homogeneous Properties Words that describe matter (adjectives) Physical Property - a property that can be observed and measured without changing the substance. Examples: Chemical Property - a property that can only be observed by changing the type of substance. Examples: States of matter Solid- matter that can not flow and has definite volume. Liquid- definite volume but takes the shape of its container (flows). Gas- a substance without definite volume or shape and can flow. Vapor- a substance that is currently a gas but normally is a liquid or solid at room temperature. States of Matter Definite Definite Volume? Shape? Solid Liquid Gas YES YES NO Expansion w/ Temp. increase Compressible? YES Small Expans. NO NO Small Expans. NO NO Large Expans. YES Condense Freeze Melt Solid Deposition Liquid Sublimation Boil Gas States of Matter Plasma: Occurs at high temperature low pressure Formed when electrons separate from nucleus of gases Most common state of matter in the universe Changes in Matter Physical Changes & Chemical Changes Physical Changes These alter the appearance of matter without changing its composition. Examples? Changes of the state (or phase) of matter is a physical change The temperature & pressure at which matter changes phase are important physical properties These are melting & boiling points Another Way to Change States Pressure For some substances it will turn solids to liquids For others it will turn liquids to solids Ex: Silly Putty Will turn gas to liquid EX: Ice Skating Compressor in refrigerator and AC Will turn gas to solid Formation of Dry Ice Chemical Changes Occur when one or more substances react and form a new substance These are also called chemical reactions Products of the chemical reactions ALWAYS have different properties than the original materials Evidence of Chemical Reactions: Formation of a gas Formation of a solid Drastic color change Energy changes – temp. changes or formation of light Exothermic vs. Endothermic Reactions Change in the smell of a substance Conservation of Mass Mass can not be created or destroyed in ordinary chemical changes All the mass can be accounted for Mass at the start = mass at end So the total mass of the products should equal the total mass of the reactants Antoine Lavoisier Considered the father of modern Chemistry Discovered the Law of Conservation of Mass (also called the Law of Conservation of Matter) Did experiments on reactions. Mixtures A physical combination of two or more substances Heterogeneous - you can see the individual substances that make it up. Ex: Chocolate chip cookie dough, gravel, soil. Homogeneous- you cannot see the individual substances that make it up, it always has a single phase. Ex: Kool-aid, air. Solutions Another name for homogeneous mixture Can occur between any state of matter. Examples: Liquid Solid Gas Separating mixtures Only physical changes - no new matter formed Filtration- separate solids from liquids with a barrier Distillation- separate because of different boiling points Heat mixture Catch purified vapor in cooled area Chromatography- different substances are attracted to paper or gel, so move at different speeds Crystallization – pure solids form when solid particles come out of solution (make crystals) Elements & Compounds Section 3.4 Elements Simplest pure substance Cannot be broken down into simpler matter by normal physical or chemical means All one kind of atom Each one has a unique name and symbol. In the symbol the first letter is always capitalized and the remaining letter(s) are lowercase. There are 91 naturally occurring elements Who was given credit for organizing them into a table? Dmitri Mendeleev Compounds Chemical combinations of two or more different elements When they are broken down, the pieces have completely different properties than the compound. For example: Salt Most of the substances we work with are compounds Chemical Symbols Used to write chemical formulas You know it is a formula because there will be more than one capital letter Subscripts tell us how many of each atom in the formula. H2O C3H8 HBrO3 Laws of Compounds By accounting for the mass of all matter there are a couple of laws that govern how compounds are created. Law of Definite Proportions Law of Multiple Proportions Law of Definite Proportions Each compound has a specific ratio of elements by mass For example – the chemical formula for water is always H2O You can also see its ratio by mass: It is always a whole # ratio Water is always 8 grams of oxygen for each gram of hydrogen This does not change, no matter where you are on Earth! (Or in the universe for that matter) Law of Def. Prop. Continued… This law can be verified by determining the percent by mass of the elements in a compound: mass of element Percent by mass (%) = x100 mass of compound Example: A compound contains 52.46 g of iron and 22.54 g of oxygen. What is the percent composition of oxygen? 30.05 % Oxygen Law of Multiple Proportions The textbook states that when the same elements combine to make different compounds, they combine in small whole number ratios. You will NOT get fractions of elements Examples: CuCl vs. CuCl2 CH4 vs. C2H6 Review What makes a form of matter a substance? What are some examples? How do you distinguish between chemical and physical properties? What is the difference between a physical change and a chemical change? Give one example of each. Why is a solution a homogeneous mixture? What is one way to separate a homogeneous mixture? Explain how you would tell that NaCl is a compound but Na is an element. Explain how all compounds obey the laws of definite and multiple proportions.