* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Trends in Biomedical Science
Nicotinic agonist wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Psychopharmacology wikipedia , lookup
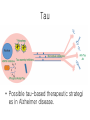
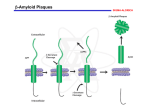
Trends in Biomedical Science Prospective Treatments for Alzheimer’s For each of the following think about: 1. What the treatment does 2. Why it may be helpful There is no treatment that can cure Alzheimer’s or even halt its progress, but there are some medications that can temporarily alleviate the cognitive deficits associated with it. In just about half of all patients who respond to these medications, the reduction in symptoms begins 3 to 6 months after the start of treatment, and lasts an average of 6 to 12 months. But the efficacy of these medications is hard to assess, because of variations in their dosage, in how long they are administered, in the route by which they are administered, and so on. The class of drugs most often used to treat Alzheimer’s are the anticholinesterases, or cholinesterase inhibitors. Another medication, memantine, works by inhibiting the brain’s glutamate receptors. Like the anticholinesterases, it is only moderately effective and has no great impact on the course of the disease. These medications appear to be more effective when taken early, in the mild to moderate stages of the disease. Anticholinesterases work by preventing a class of enzymes known as cholinesterases from breaking down acetylcholine. Acetylcholine is a neurotransmitter that is especially important for memory and attention and is produced by neurons that are easily affected by Alzheimer’s. Anticholinesterase medications thus increase the concentration of this neurotransmitter in the brain. The first anticholinesterase medication to be approved as a treatment for AD was tacrine, in 1994. But tacrine had bad side effects, especially on the liver and the digestive tract. Between 1997 and 2001, three new second-generation cholinesterase inhibitors came to market that were just as effective as tacrine but better tolerated, and tacrine was taken off the shelves. But they still have mild to moderate digestive side effects (nausea, vomiting, loss of appetite, diarrhea) in 10 to 20% of patients. Also, all three can become less effective over time, because the neurons of people with Alzheimer’s produce less and less acetylcholine. The fourth medication now commonly prescribed for Alzheimer’s is memantine. Memantine is classified as an antiglutamate. In 2003 it was the first medication approved for use in people with moderate to severe Alzheimer’s symptoms. As Alzheimer’s progresses, excessive amounts of glutamate are produced in certain parts of the brain, thus overstimulating one class of glutamate receptors, the NMDA receptors, and making the glutamate toxic to the neurons. Memantine acts as an NMDA-receptor antagonist, taking the place of glutamate and thus reducing the toxic effects that glutamate has when it is in excess. Memantine is generally well tolerated; its undesirable side effects (dizziness, headaches, etc.) are reported by fewer than 10% of all patients. Amyloid beta Amyloidogenic processing of amyloid precursor protein (APP) by BACE1 and g-secretase. The figure depicts the principal proteolytic processing steps of APP leading to the production of 40–42-residue amyloid b (Ab) peptide, the subsequent steps ultimately culminating in compaction and deposition of the peptide in b-amyloid plaques in brain of AD patients (and transgenic AD mouse models), and the primary point of intervention by the different therapeutic antiamyloid approaches. Future treatments now being developed for Alzheimer’s target the beta-amyloid peptide that is responsible for senile plaques and the tau protein that is the source of neurofibrillary tangles. Researchers are trying to block the harmful effects attributed to beta-amyloid by inhibiting its formation, by breaking down amyloid plaques that have already formed, and by modifying the abnormal form of the tau protein. One approach being explored for breaking plaques down is immunotherapy, in other words, a vaccine against Alzheimer’s. Research is being done on two different forms of vaccination: active and passive. In active vaccination, Alzheimer’s patients are injected with an antigen so that their immune systems start to produce antibodies that attack the amyloid plaques. In passive vaccination, the antibody against beta-amyloid is injected directly. In 1999, active vaccination was shown to be effective in transgenic mice. After vaccination, these mice produced antibodies against the beta-amyloid peptide, had lower levels of beta-amyloid in their blood, and showed improved cognitive function. The first human trials of this vaccine began in 2001 but were discontinued one year later because of serious side effects (encephalitis) in 18 of the 300 subjects who had been vaccinated (6%). Follow-up showed that in some of these subjects, there was a decrease in amyloid deposits together with modest improvement in cognitive function. One example of research on passive vaccination was a set of clinical trials conducted in 2008 with the monoclonal antibody bapineuzumab, which binds specifically to the beta-amyloid protein. Here too, a slight improvement in symptoms was observed, but with some significant side effects. Tau • Possible tau-based therapeutic strategi es in Alzheimer disease. A loss of tau function might be overcome with microtubule-stabilizing agents or inhibitors of tau hyperphosphorylation and/or acetylation. Potentially toxic tau oligomers or fibrils might be prevented by inhibitors of tau multimeric assembly. Inhibition of HSP90 and the resulting elevation of the chaperones HSP70/HSP40 may increase proteasomal degradation of hyperphosphorylated tau. Misfolded tau multimers might be cleared through enhancement of macroautophagy. Finally, misfolded tau species may be released from cells and internalized by nearby neurons, thereby “seeding” the formation of pathological tau in the recipient cell. If confirmed, this spreading of tau pathology might be inhibited by antibodies that bind misfolded tau in the brain interstitial fluid. To block the build-up of defective tau proteins in the neurons, the most promising drugs involve two well known compounds that have been used in medications for many years. The first of these drugs is methylthioninium chloride (commonly known as methylene blue), a molecule first synthesized in 1876 and used since then to treat malaria, urinary infections, and many other conditions. In clinical trials, methylene blue appeared to stabilize the cognitive decline of 321 subjects in the early to middle stages of Alzheimer’s. After 19 months of taking a moderate dose three times per day, the subjects in the active-drug group had still shown no further decline, whereas in the placebo-drug group, the subjects’ decline had continued. Moreover, brain imaging showed that in some of the subjects’ brains, this drug had been active in the areas where defective tau proteins were most abundant. The second of these drugs, latrepirdine, was originally developed and marketed as an antihistamine in Russia. It has many chemical properties and appears to have a beneficial effect on the mitochondria of the neurons of people with Alzheimer’s. A trial involving Russian subjects with Alzheimer’s, published in the medical journal The Lancet in 2008, showed that, compared with members of the placebo-drug group, members of the group that received latrepirdine showed better memory and fewer behavioral problems. But the results of a Phase 3 clinical trial involving patients in North America, South America, and Europe, published early in 2010, did not show the same effectiveness. Though these results are disappointing, they do not necessarily mean that latrepirdine will not eventually be recognized as effective. That is what happened with memantine, for which some trials also produced negative results. The US Food and Drug Administration (FDA), the body that approves drugs for use in the United States, will generally grant approval when two-thirds of the results are positive. Researchers are exploring many ways to try to find medications for Alzheimer’—for example, any drug that could improve the effectiveness of a certain type of microglia that can effectively eliminate amyloid plaques. But in addition to medications, there are a wide variety of “complementary", nonmedical therapies that can improve or slow the progress of certain symptoms. Many of these therapies attempt to stimulate patients’ remaining faculties so as to improve wellness and quality of life. One example is cognitive remediation, a computerassisted therapy that stimulates abilities needed to perform activities of daily life—abilities such as attention, memory, language, and hand-eye coordination. Another is reminiscence therapy, which uses items such as photographs and music to evoke memories from Alzheimer’s patients’ past and get them to talk about it. Still another therapy uses multiple sensory experiences in a peaceful setting to improve Alzheimer’s patients’ mood while reducing agitation and apathy. Other approaches are based on using art, music, pets, and other methods to provide emotional stimulus. • To date, research on vitamins (B, C, and E) and other substances such as ginkgo biloba, folic acid, and selenium has not provided any conclusive evidence that they are effective in preventing Alzheimer’s or slowing its progress. (however see Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment PNAS 23 110 (23) 9523-9528.)