* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Loop diuretics
Environmental persistent pharmaceutical pollutant wikipedia , lookup
Neuropharmacology wikipedia , lookup
Environmental impact of pharmaceuticals and personal care products wikipedia , lookup
Oral rehydration therapy wikipedia , lookup
Drug interaction wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
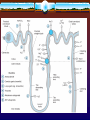
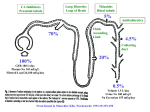
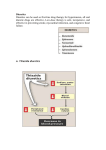
Diuretics Diuretic A "diuretic" is an agent that increases urine volume, while a "natriuretic" causes an increase in renal sodium excretion. Because natriuretics almost always also increase water excretion, they are usually called diuretics. Renal Tubule Transport Mechanisms: Approximately 16 to 20 percent of the blood plasma entering the kidneys is filtered from the glomerular capillaries into the Bowman's capsule. The filtrate, although normally free of proteins and blood cells, does contain most low-molecular-weight plasma components in approximately the same concentrations as are found in the plasma. These include glucose, sodium bicarbonate, amino acids, and other organic solutes as well as electrolytes, such as Na+, K+, and Cl-. Regulation of Fluid and Electrolytes by the Kidneys A. Proximal convoluted tubuleNormal - Approximately 66% of total sodium ions (Na+), 85% of the filtered NaHCO3), 65% of the K+, 60% of the water, and virtually all of the filtered glucose and amino acids are reabsorbed in the proximal tubule. Sodium bicarbonate reabsorption is initiated by the action of Na+\H+ exchange, allow the Na+ to enter the cell from tubular lumen in one for one exchange with a proton usually H+ from inside the cells. Proton secreted in the lumen combine with bicarbonate to form carbonic acid (H2CO3), H2CO3 is dehydrated in order to be transported into CO2 +H2O which cross the membranes, and this reaction is catalyzed by carbonic anhydrase enzyme (CA). B. .LOOP OF HENLE Descending loop of Henle The proximal tubule empties into the thin descending limb of Henle's loop. Water is extracted from the descending limb of this loop by osmotic forces found in the hypertonic medullary interstitium. And not participate in salt reabsorption . As the DLOH freely permeabile to water and not to solutes until it reaches benet of LOH ( maxim. Concentrated urine ). The thick ascending : The thick ascending limb (TAL) of the loop of Henle actively reabsorbs NaCl from the lumen (about 25% of the filtered sodium), The thick ascending limb is nearly impermeable to water. Salt reabsorption in the TAL therefore dilutes the tubular fluid, and it is called a "diluting segment." The NaCl transport system in the luminal membrane of the TAL is a Na+/K+/2Cl- cotransporter . This transporter is selectively blocked by diuretic agents known as "loop" diuretics. C- Distal convulated tubule Only about 10% of the filtered NaCl is reabsorbed in the distal convoluted tubule (DCT). This segment is relatively impermeable to water , and NaCl reabsorption further dilutes the tubular fluid. This Na+ and Cl- cotransport is blocked by Thiazide-diuretics. Ca2+ is actively reabsorbed by the DCT epithelial cell via an apical Ca2+ channel and basolateral Na+/Ca2+ exchanger. This process is regulated by parathyroid hormone. D- Convoluting Collecting tubule (CCT):(( Na+ exchange for K+, H+) The collecting tubule is the most important site of K+ secretion by the kidney and the site at which virtually all diuretic-induced changes in K+balance occur . The collecting tubule is responsible for only 2-5% of NaCl reabsorption by the kidney. As it is the final site of NaCl reabsorption, the collecting tubule is responsible for tight regulation of body fluid volume and for determining the final Na+ concentration of the urine. Reabsorption of Na+ via the epithelial Na channel (ENaC) and its coupled secretion of K+ is regulated by aldosterone. Carbonic Anhydrase Inhibitors Acetazolamide Mechanism of action: Carbonic anhydrase is present in many nephron sites, but the predominant location of this enzyme is the luminal membrane of the PCT where it catalyzes the dehydration of H2CO3. By blocking carbonic anhydrase, the exchange of Na+ to H+ will be decreased and result in mild diuresis ,in addition NaHCO3 is retained in the lumin with elevation of urinary PH. The loss of HCO3 and retained of H+ result in metabolic acidosis . s Pharmacodynamics Inhibition of carbonic anhydrase activity profoundly depresses 85% of the HCO3- reabsorption in the PCT., and causes significant HCO3- losses . ,the fact that HCO3- depletion leads to enhanced NaCl reabsorption by the remainder of the nephron, the diuretic efficacy of acetazolamide decreases significantly with use over several days and hyperchloremic metabolic acidosis occur. At present, the major clinical applications of acetazolamide involve carbonic anhydrase-dependent HCO3- and fluid transport at sites other than the kidney. The ciliary body of the eye secretes HCO3from the blood into the aqueous humor. Likewise, formation of cerebrospinal fluid by the choroid plexus involves HCO3- secretion, they are similarly inhibited by carbonic anhydrase inhibitors. Therapeutic uses: A.Glaucoma : The most common use of acetazolamide is to reduce the elevated intraocular pressure of open-angle glaucoma. Acetazolamide decreases the production of aqueous humor, probably by blocking carbonic anhydrase in the ciliary body of the eye. It is useful in the chronic treatment of glaucoma but should not be used for an acute attack; pilocarpine is preferred for an acute attack because of its immediate action. B.Urinary alkalinization C. Metabolic alkalosis . D. Acute mountain sickness. Side effects A- Hyperchloremic metabolic acidosis B. Renal stones . : Phosphaturia and hypercalciuria occur during the bicarbonaturic response to inhibitors of carbonic anhydrase. Calcium salts are relatively insoluble at alkaline pH, which means that the potential for renal stone formation from these salts is enhanced. C. Renal potassium wasting . Potassium wasting can occur because Na+ presented to the collecting tubule is partially reabsorbed, and thus enhancing K+ secretion. D. Other toxicities. Drowsiness and paresthesias are common following large doses of acetazolamide. Carbonic anhydrase inhibitors may accumulate in patients with renal failure, leading to nervous system toxicity. Hypersensitivity reactions (fever, rashes, bone marrow suppression, and interstitial nephritis) may also occur. Loop diuretics (furosemide bumetanide and torsemide and ethacrynic acid). mechanism of action Mechanism of action . These drugs inhibit the luminal Na+/K+/2Clco-transporter in the thick ascending limb of Henle's loop. By inhibiting this transporter, the loop diuretics reduce the reabsorption of NaCl and also diminish the lumen-positive potential that comes from K+ recycling . This positive potential normally drives divalent cation reabsorption in the loop and by reducing this potential, loop diuretics cause an increase in Mg2+ and Ca2+ excretion. Prolonged use can cause significant hypomagnesemia in some patients. Since the ALOH responsible for reabsorption of 25-30% of filterd Nacl, and the downstream sites cannot able to compensate for this increased Na+ load, the loop diuretics are the most efficacious agents . In addition to their diuretic activity, loop agents have direct effects on blood flow through several vascular beds by inducing the synthesis of prostaglandins, both furosemide and ethacrynic acid have also been shown to reduce pulmonary congestion and left ventricular filling pressures in heart failure before a measurable increase in urinary output occurs. Pharmacokinetics The loop diuretics are rapidly absorbed. They are eliminated by the kidney by glomerular filtration and tubular secretion. Absorption of oral torsemide is more rapid (1 hour) than that of furosemide (2-3 hours) and is nearly as complete as with intravenous administration. The duration of effect for furosemide is usually 2-3 hours and that of torsemide is 4-6 hours. Half-life depends on renal function. Clinical Indications : The most important indications : Acute pulmonary edema, other edematous conditions, hyperkalemia, acute renal failure, and anion overdose(toxic ingestions of bromide, fluoride, and iodide, which are reabsorbed in the thick ascending limb). Side effects. A. hypokalemic metabolic alkalosis . By inhibiting salt reabsorption in the TAL, loop diuretics increase Na delivery to the collecting duct. Increased delivery leads to increased secretion of K+ and H+ by the duct, causing hypokalemic metabolic alkalosis .This toxicity is a function of the magnitude of the diuresis and can be reversed by K+ replacement and correction of hypovolemia. B. Ototoxicity. Loop diuretics occasionally cause dose-related hearing loss that is usually reversible. It is most common in patients receiving other ototoxic agents such as aminoglycoside antibiotics. . C. Hyperuricemia. Loop diuretics compete with uric acid secretion in the proximal tubule. . D.Hypomagnesmia . E.Allergic reaction Except for ethacrynic acid, the loop diuretics are sulfonamides. Therefore skin rash, eosinophilia and, less often, interstitial nephritis are occasional side effects of these drugs. This toxicity usually resolves rapidly after drug withdrawal. Loop diuretics can cause sever dehydration The thiazide diuretics are the most widely used of the diuretics drugs, thiazides inhibit NaCl transport predominantly in the DCT by different mechanisms. The prototypical thiazide is hydrochlorothiazide. Pharmacokinetics : All of the thiazides can be administered orally. All of the thiazides are secreted by the organic acid secretory system in the proximal tubule and compete with the secretion of uric acid by that system. As a result, thiazide use may blunt uric acid secretion and elevate serum uric acid level. . Pharmacodynamics Thiazides inhibit NaCl reabsorption from the luminal side of epithelial cells in the DCT by blocking the Na+/Cl- transporter , as aresult these drugs increase the concentration of Na ana Cl- in the tubular fluid . The increased Na+ in the filtrate arriving in distal tubule , more K- will also exchange for Na+, thus prolonged use of thiazide result in continous loss of K+ from the body . In contrast to the situation in the TAL, where loop diuretics inhibit Ca2+ reabsorption, thiazides actually enhance Ca2+ reabsorption. Thiazides are useful in the treatment of kidney stones caused by hypercalciuria. The action of thiazides depends in part on renal prostaglandin production, therefore with continues use there is a continuous hypotensive effect resulting from reduced pvr caused by relaxation of arteriolar smooth muscles. The actions of thiazides can also be inhibited by NSAIDs under certain conditions Clinical Indications : The major indications for thiazide diuretics are (1) hypertension, (2) heart failure, (3) nephrolithiasis due to idiopathic hypercalciuria, and (4) nephrogenic diabetes insipidus, because thiazide have ability to produce hyperosmollar urine Side effects A.hypokalemia. B- Hyponatremia.C- Hypercalcemia D- Hyperuricemia. Compete with uric acid secretion. E-Hyperglycemia may occur in patients who are overtly diabetic or who have even mildly abnormal glucose tolerance tests. The effect is due to both impaired pancreatic release of insulin and diminished tissue utilization of glucose. F.Hyperlipidemia :Thiazides cause a 5-15% increase in total serum cholesterol and low-density lipoproteins (LDL). These levels may return toward baseline after prolonged use. G. Allergic reaction The thiazides are sulfonamides and share cross-reactivity with other members of this chemical group. Photosensitivity or generalized dermatitis occurs rarely. F. Other toxicities.Weakness, fatigability, and paresthesias similar to those of carbonic anhydrase inhibitors may occur. Impotence has been reported but is probably related to volume depletion. Volume depletion can cause orthostatic hypotension Potassium –sparing diuretics mechanism of action POTASSIUM-SPARING DIURETICS. These diuretics prevent K+ secretion by antagonizing the effects of aldosterone at the late distal and cortical collecting tubules. Inhibition may occur by direct pharmacologic antagonism of mineralocorticoid receptors (spironolactone) or by inhibition of Na+ influx through ion channels in the luminal membrane (amiloride, triamterene). Pharmacokinetics: Overall, spironolactone has a rather slow onset of action, requiring several days before full therapeutic effect is achieved. Amiloride and triamterene are direct inhibitors of Na+ influx in the CCT. Triamterene is metabolized in the liver, and excreted by the kidney, it has a shorter half-life and must be given more frequently than amiloride . Pharmacodynamics Potassium-sparing diuretics reduce Na+ absorption in the collecting tubules and ducts. Na+ absorption (and K+ secretion) at this site is regulated by aldosterone, as described above. Spironolactone binds to aldosterone receptors and act as competitive antagonist toaldesteron. Amiloride and triamterene do not block the aldosterone receptor but instead directly inhibits Na+ reabsorption in CCT. Since K+ secretion is coupled with Na+ entry in this segment, these agents are also effective potassium-sparing diuretics. Similar effects are observed with respect to H+ handling by the intercalated cells of the collecting tubule, in part explaining the metabolic acidosis seen with aldosterone antagonists). Clinical Indications . Potassium-sparing diuretics are most useful in states of mineralocorticoid excess or hyperaldosteronism (also called aldosteronism), due either to primary hypersecretion (Conn's syndrome, ectopic adrenocorticotropic hormone production) or to secondary hyperaldosteronism (evoked by heart failure, hepatic cirrhosis, nephrotic syndrome, or other conditions associated with diminished effective intravascular volume). Toxicity A.Hyperkalemia Unlike other diuretics, K+-sparing diuretics can cause mild, moderate, or even lifethreatening hyperkalemia . The risk of this complication is greatly increased by renal disease (in which maximal K+ excretion may be reduced) or by the use of other drugs that reduce renin (beta- blockers, NSAIDs) or angiotensin II activity (angiotensin-converting enzyme inhibitors, angiotensin receptor inhibitors B. metabolic acidosis ; By inhibiting H+ secretion in parallel with K+ secretion. C. Gyencomastia : Gynecomastia, impotence, and benign prostatic hyperplasia have all been reported with spironolactone. D. Acute renal failure .The combination of triamterene with indomethacin has been reported to cause acute renal failure. This has not been reported with other K+-sparing diuretics. E. Kidney stones; Triamterene is only slightly soluble and may precipitate in the urine, causing kidney stones. Osmotic Diuretics (Manitol). The proximal tubule and descending limb of Henle's loop are freely permeable to water . Any osmotically active agent that is filtered by the glomerulus but not reabsorbed such as mannitol and urea cause water to be retained in these segments. If the substance that is filtered subsequently undergoes little or no reabsorption, then the filtered substance will cause an increase in urinary output and promotes a water diuresis. Pharmacokinetics : Osmotic diuretics are poorly absorbed, which means that they must be given parenterally. If administered orally, mannitol causes osmotic diarrhea. Mannitol is not metabolized and is excreted by glomerular filtration within 30-60 minutes, without any important tubular reabsorption or secretion. Pharmacodynamics : Osmotic diuretics have their major effect in the proximal tubule and the descending limb of Henle's loop. The presence of a nonreabsorbable solute such as mannitol prevents the normal absorption of water by interposing a countervailing osmotic force. As a result, urine volume increases. The increase in urine flow rate decreases the contact time between fluid and the tubular epithelium, thus reducing Na+ as well as water reabsorption. The resulting natriuresis is of lesser magnitude than the water diuresis, leading eventually to excessive water loss and hypernatremia. Clinical Indications A.To increase urine. Osmotic diuretics are used to increase water excretion in preference to sodium excretion. This effect can be useful when avid Na+ retention limits the response to conventional agents. It can be used to maintain urine volume and to prevent anuria that might otherwise result from presentation of large pigment loads to the kidney (eg, from hemolysis or rhabdomyolysis). B. Reduction of intracranial and intraoculal pressure. Osmotic diuretics alter Starling forces so that water leaves cells and reduces intracellular volume. This effect is used to reduce intracranial pressure in neurologic conditions and to reduce intraocular pressure before ophthalmologic procedures. A dose of 12 g/kg mannitol is administered intravenously. Toxicity. A. Extracellular volume expansion Mannitol is rapidly distributed in the extracellular compartment and extracts water from cells. Prior to the diuresis, this leads to expansion of the extracellular volume and hyponatremia. This effect can complicate heart failure and may produce florid pulmonary edema. Headache, nausea, and vomiting are commonly observed in patients treated with osmotic diuretics. B. Dehydration, Hyperkalemia,and hypernatremia . Excessive use of mannitol without adequate water replacement can ultimately lead to severe dehydration, free water losses, and hypernatremia. As water is extracted from cells, intracellular K+ concentration rises, leading to hyperkalemia. ADH Antagonists ADH regulates water balance in the body (controlled in the hypothalamus , excreted by the posterior pituitary, controls aquaporins). ADH antagonists block the ADH receptors in the kidneys. Inhibition of ADH receptors causes excretion of free water without electrolyte loss (aquaresis). • Conivaptan • Lixivaptan • Tolvaptan ADH Antagonists • Conivaptan • Lixivaptan • Tolvaptan 25-42 Preferred Treatment © 2012 The McGraw-Hill Companies, Inc. All rights reserved. THANKS FOR YOUR ATTENTION !!!