* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Health Care Statistics
Survey
Document related concepts
Transcript
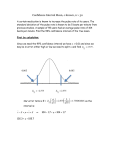
Statistics for Health Care Biostatistics Phases of a Full Clinical Trial • Phase I – the trial takes place after the development of a therapy and is designed to determine doses, strengths and safety – it is preexperimental – there is no control group • Phase II – the trial is looking for evidence of effectiveness – it is pre-experimental or quasiexperimental and similar to a pilot test – it seeks to determine feasibility of further testing and signs of potential side effects Phases of a Full Clinical Trial • Phase III – it is a full experimental test of the therapy with randomly assigned treatment and control groups. It is designed to determine whether the intervention is more effective than a standard treatment. It may be referred to as and RCT or randomized clinical trial and often studies large groups from multiple sites. Phases of a Full Clinical Trial • Phase IV – it occurs after the decision to adopt an innovative therapy and involves study of its long-term consequences –both side effects and benefits. It rarely requires true experimental design. Prevalence Studies • Prevalence studies are a type of descriptive study that come from the field of epidemiology. They are conducted to determine the prevalence rate of some condition. They are cross-sectional designs that obtain data from one population at risk for the condition Prevalence Studies • The formula for a prevalence rate (PR) is: # of cases with the condition at a given point in time _____________________________________ x K # in the population at risk for being a case Example: 80 cases ____________ x 100 (# we want the PR established for) = 16 per 100 500 at risk Incidence Studies • Incidence studies measure the frequency of developing new cases. This requires a longitudinal design, because we need to determine who is at risk for becoming a new case. Incidence Studies • The formula for an incidence rate (IR) is: # of new cases with the condition over a given period of time _____________________________________ # in the population at risk for being a new case Example: 21 new cases (in one year since original count) ____________ x 100 = 5 per 100 420 at risk (from previous example: 500-80 =420 now at risk x K Relative Risk • Relative risk (RR is the risk of becoming a case if in one group compared to being in another group. • Example: If the risk of becoming a new case was 7 per hundred in men and 14 per hundred in women, we would divide the rate of women by the rate of men, and find that women were twice as likely to become a new case over a year’s time. Relative Risk • Relative Risk can also be used in examining new therapies. • Relative Risk is the ratio of an outcome (such as PONV) in the treatment group (those receiving a bolus of fluid before induction) to the rate of the outcome (PONV) in the controls. An RR of < 1.0 means the experimental group had less (PONV) than the control group. An RR of > 1.0 would mean that the experimental group had more (PONV) than did the controls. Developing Screening/Diagnostic Instruments • Goal is to establish a cutoff point that balances sensitivity and specificity Positive Predictive Value • The Positive Predictive Value (PPV) - the precision rate, or the posttest probability of disease - is the proportion of patients with positive test results who are correctly diagnosed. It indicates the probability with which a positive test might show the disease or condition being tested for. It is a measure of the diagnostic test. A low number means a high “false positive” rate. • • PPV = • • No. of true positives No. of true positives + No. of false positives (total who test positive) Negative Predictive Value • The Negative Predictive Value (NPV) is the proportion of patients with negative test results who are correctly diagnosed. (They do not have the disease or condition.) • NPV = No. of true negatives • No. of true negatives + No. of false negatives • (total who test negative) • Criteria for Assessing Screening/Diagnostic Instruments • Sensitivity: the instrument’s ability to correctly identify a “case”—i.e., to diagnose a condition • Specificity: the instrument’s ability to correctly identify “noncases”, that is, to screen out those without the condition EMPIRICAL Test Outcome EVIDENCE of CONDITION True False Positive True positive False Positive (Type I error) Positive Predictive Value Negative False negative (Type II error) True negative Negative Predictive Value Sensitivity Specificity BOWEL CA Fecal Occult Blood Test (POSITIVE ON COLONOSCOPY and BIOPSY) True False Positive True positive = 2 False Positive = 18 PPV 2/20 = 10% Negative False negative =1 (Type II error) True negative = 182 NPV 182/183 = 99.5% Sensitivity 2/ (2+1) = 66.7% Specificity 182/ (18 +182) = 91% Information from Slide 19 • Looking at the slides you can see that the large number of false positives and the low number of false negatives indicate that the occult blood test is poor at confirming colon cancer. However, it is good as a screening test since 99.5 % of the negative tests will be correct and the sensitivity indicates that further testing needs to be done on at least 66.7% of those testing positive. Confidence Interval • The Confidence Interval (CI) is the range of values around a sample mean that are calculated to contain the population mean with a 95% (or 99%) degree of certainty. Many researchers believe that these values give more clinical data than do p values. Confidence Interval • The POINT ESTIMATE is a single point estimated to be the mean for the population, based on a sample. When a confidence interval (CI) is reported, it is an INTERVAL ESTIMATE, a range of values that estimate the mean for a population. If a math test was given to a sample of nursing students and there was a mean of 55, then 55 would be the point estimate. If the 95% CI was reported as 52-57, there would be a 95% chance that the true mean of all nursing students would be between 52-57. • Confidence Interval • The STANDARD ERROR OF THE MEAN is used to calculate the CI. The standard error of the mean is the error that is due to sampling. Each sample mean is likely to deviate somewhat from the true population mean. The SEM is the difference between the sample mean and the unknown population mean. It is the margin of error to allow for when estimating the population mean from a random sample. It allows for chance errors. It is calculated as follows: • SD (of the sample) • Square root of the No. of people in the sample Confidence Interval • If the SEM is 3 and the mean is 100 and you want a confidence interval of 95% - that is between -1.96 and +1.96 SD from the mean, you would use the following formula: X + (1.96 x SEM) = CI • • 100 + (1.96 x 3) = 100 + 5.88 • We can say, with 95% confidence, that the population mean would fall between 94.12 and 105.88 • Confidence Interval • For a 68% CI, add the standard error of the mean (SEM) to the mean, then subtract it from the mean. For a 95% CI , multiply the SEM by 1.96, then add and subtract. For a 99% CI, multiply by 2.58, then add and subtract. Note: The intervals obtained by using these multipliers are only precise with large numbers (60 or more cases).