* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Catalyst – September, 7(1+1) 2009 - stroh
Survey
Document related concepts
Transcript
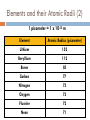
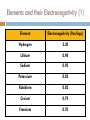
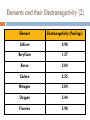
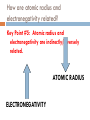
Catalyst – February 1s22s1, 2011 1. 2. Define trend. Define radius. Today’s Agenda Catalyst Review Periodic Table Basics Worksheet Periodic Trends: Notes and Work Time Exit Question HW: ANSWER CHAPTER 6 ASSESSMENT QUESTIONS 31, 49, 56, 69, 73 Today’s Objectives SWBAT describe the periodic trends for valence electrons, atomic radius, and electronegativity. Reward Vote rd (3 Period) Donuts Oreos Chocolate Chip/Sugar Cookies Granola/Fruit Other (tell me what you’d like!) PERIODIC TRENDS!!! VALENCE ELECTRONS (CHECK!) ATOMIC RADIUS ELECTRONEGATIVITY Valence Electrons Don’t forget! Valence electrons are the electrons in the outermost energy level. Let’s look at Bohr Models… http://www.chemicalelements.com/show/electronconfig.html Valence Electrons – Right on your PT Trend for Valence Electrons Key Point #1: Number of valence electrons increases from 1-8 as you go right across the periodic table. What is Atomic Radius? Key Point #2: Atomic radius is how big an atom is and is also known as atomic size. What is Electronegativity? Key Point #3: Electronegativity is the ability of an atom to attract electrons to itself in a chemical bond. How much an atom wants electrons Graphing Atomic Radius (Size) Draw 2 line graphs X-axis: Element Y-axis: Atomic Radius (picometers) Elements and their Atomic Radii (1) 1 picometer = 1 x 10-12 m Element Atomic Radius (picometer) Hydrogen 37 Lithium 152 Sodium 186 Potassium 227 Rubidium 248 Cesium 265 Elements and their Atomic Radii (2) 1 picometer = 1 x 10-12 m Element Atomic Radius (picometer) Lithium 152 Beryllium 112 Boron 85 Carbon 77 Nitrogen 75 Oxygen 73 Fluorine 72 Neon 71 Atomic Size – Graph 1 Atomic Radius (picometer) 350 300 250 200 150 100 50 0 0 1 H 2 Li 3 Na 4 K Element 5 Rb 6 Cs 7 Atomic Size – Graph 2 Atomic Radius (picometer) 160 140 120 100 80 60 40 20 0 0 Li 2 Be B C4 6 N O Element F 8 Ne 10 What trend(s) do you notice? TRENDS FOR ATOMIC RADIUS Key Point #4: Atomic size INCREASES as you go DOWN the periodic table and DECREASES as you go LEFT TO RIGHT across the periodic table. Practice Problems Rank the following elements in order of increasing atomic size based on location on the periodic table (smallest to biggest) Fr, Sc, P, Pd P < Sc < Ps < Fr F, As, Tl, S F < S < As < Tl Graphing Electronegativity Draw 2 line graphs X-axis: Element Y-axis: Electronegativity (Paulings) Elements and their Electronegativity (1) Element Electronegativity (Paulings) Hydrogen 2.20 Lithium 0.98 Sodium 0.93 Potassium 0.82 Rubidium 0.82 Cesium 0.79 Francium 0.70 Elements and their Electronegativity (2) Element Electronegativity (Paulings) Lithium 0.98 Beryllium 1.57 Boron 2.04 Carbon 2.55 Nitrogen 3.04 Oxygen 3.44 Fluorine 3.98 Electronegativity Graph 1 2.5 Electronegativity 2 1.5 1 0.5 0 0 1 2 3 4 Element 5 6 7 8 Electronegativity Graph 2 Electronegativity 4.5 4 3.5 3 2.5 2 1.5 1 0.5 0 0 2 4 Element 6 8 What trend(s) do you notice? Electronegativity Trends TRENDS FOR ELECTRONEGATIVITY Key Point #5: Electronegativity DECREASES as you go DOWN the periodic table and INCREASES as you go LEFT TO RIGHT across the periodic table. Practice Problems Rank the following elements in order of increasing electronegativity based on location on the periodic table (smallest to biggest) Mg, Sr, Be, Ra Ra < Sr < Mg < Be Cl, Si, Al, S, P Al < Si < P < S < Cl So as you go LEFT to RIGHT… TO SUM IT UP: As you move left to right across the periodic table, positive charge increases so… ELECTRONEGATIVITY INCREASES Therefore, ATOMIC RADIUS DECREASES So as you go from TOP to BOTTOM… TO SUM IT UP: As you go from top to bottom on the periodic table, energy levels (shells) increase so… ATOMIC RADIUS INCREASES Therefore, ELECTRONEGATIVITY DECREASES How are atomic radius and electronegativity related? Key Point #5: Atomic radius and electronegativity are indirectly/inversely related. ATOMIC RADIUS ELECTRONEGATIVITY Why is this relationship true? Atoms with HIGH ELECTRONEGATIVITIES hold their electrons very close! Sooooo, the atomic radius decreases High or low electronegativity? Large or small atomic size? Why is this relationship true? Atoms with LARGE RADII can’t pull on their electrons as much Soooo, ELECTRONEGATIVITY decreases! More Practice! 1. 2. 3. T or F? Atomic size decreases as you move right across the periodic table. T or F? As you move down the Periodic Table, atoms get smaller. Rank the following sets of elements in order of increasing atomic size (small big). Set A: Bh, Mn, Re, Tc Set C: Y, Ti, Sg, Ta 4. Set B: Sb, I, Ag, Ru Rank the following sets of elements in order of decreasing atomic size (big small). Set A: Cl, At, I, F, Br Set B: Te, Xe, Sn, In Set C: Rb, K, Sr, Ca More Practice! 1. 2. 3. T or F? Electronegativity decreases as you move left across the periodic table. T or F? As you move down the Periodic Table, atoms get more electronegative. Rank the following sets of elements in order of increasing electronegativity (small big). Set A: Bh, Mn, Re, Tc Set C: Y, Ti, Sg, Ta 4. Set B: Sb, I, Ag, Ru Rank the following sets of elements in order of decreasing electronegativity (big small). Set A: Cl, At, I, F, Br Set B: Te, Xe, Sn, In Set C: Rb, K, Sr, Ca Exit Question 1. 2. 3. Which element has atoms with the smallest radius: Cl, Se, P, or F? Which element has the largest electronegativity: Ag, Cu, Hg, or Zn? How are atomic radius and electronegativity related? HW: ANSWER CHAPTER 6 ASSESSMENT QUESTIONS 31, 49, 56, 69, 73