* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Atom and Light
Tight binding wikipedia , lookup
Planck's law wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Electron scattering wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Double-slit experiment wikipedia , lookup
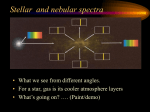
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Atomic theory wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Atom and Light Lancelot L. Kao Updated: Jan 24, 2010 Outline • • • • • • • • • Nature of Light Basic Properties of Light Three Types of Spectra Useful Application – Doppler Effect Atomic Structure Periodic Table of the Elements Interaction of Light and Matter Blackbody Radiation States/Phases of Matter 2 Nature of Light • What is the “Nature” of Light? • Observed Properties of Light – reflection, refraction, diffraction, & interference • Wave Properties – reflection, refraction, diffraction, & interference • Particle Properties – reflection, refraction • Particle-Wave Duality 3 Wave Properties of Light • Wave Properties – diffraction – Interference • Young’s Double-Slit Experiment (1801) 4 Wave Properties of Light • Electromagnetic Waves – wavelength (meter) – frequency (cycles per second = Hertz = Hz) • Speed of Light – constant within a medium – absolute speed – 3.0 x 108 m/s in vacuum Fizeau-Foucault Method (1850) 5 Electromagnetic Spectrum • Speed of Light = wavelength x frequency • C=lxn 6 Particle Properties of Light • Max Planck (1900) – Blackbody Radiation – Electromagnetic energy (radiation) is emitted in discrete, particlelike packet. • Albert Einstein (1905) – Re-interpret Planck’s result – Photoelectric Effect – Light as particles -photons • Energy of a Photon – The energy of a photon is proportional to its frequency. 7 Three Types of Spectra 8 Interaction of Light and Matter • Kirchoff’s 1st Law – A hot opaque body, such as a perfect blackbody, or a hot, dense gas produces a continuous spectrum. • Kirchoff’s 2nd Law – A hot, transparent gas produces an emission line spectrum. • Kirchoff’s 3rd Law – A cool, transparent gas in front of a source of a continuous spectrum produces an absorption line spectrum. The absorption lines in the absorption line spectrum of a particular gas occur at exactly the same wavelengths as the emission lines in the emission line spectrum of the same gas. • Types of spectra of matter can produce is dictated by the physical condition/state of the matter. 9 Doppler Effect l = wavelength shift l v lo c lo = lab wavelength V = radial velocity c = speed of light + = redshift - = blueshift • • • Christian Doppler (1842) The apparent change in wavelength or frequency of radiation due to relative motion between the source and the observer along the line-of-sight. If the relative motion between the source and the observer is moving away from each other, the observed spectral line is longer than the lab wavelength, it is called a redshift. If the relative motion between the source and the observer is moving towards each other, the observed wavelength is shorter than the lab wavelength, it is called a blueshift. 10 Doppler Effect Stationary Source Moving Source 11 An Example 12 Relativistic Doppler Effect lobserved lo v 1 c v 1 c lobserved = observed wavelenght lo = lab wavelength v = radial velocity c = speed of light + = redshift - = blueshift 13 Atomic Structure • Rutherford’s Experiment (1910) • Rutherford’s Model of the Atom • Atomic Structure – cloud of electrons – nucleus • protons • neutrons 14 Atomic Structure 15 16 Interaction of Light and Matter • Bohr Model of Hydrogen Atom – energy levels or states • Quantum Rules – Electron can only occupy at discrete energy level. – Electron can jump from one energy level to another only if it gains or loses a specific amount of energy equal to the differences of the levels. 17 Interaction of Light and Matter Electron Transitions in Hydrogen 18 Interaction of Light and Matter 19 Blackbody Radiation 20 Blackbody Radiation • Wien’s Law – The wavelength of maximum emission of a blackbody is inversely proportional to its temperature. 0.0029 lmax (m) T (K ) • Stefan-Boltzmann Law – The energy flux (power per unit area) of a blackbody is proportional to the 4th power of its temperature. 2 Flux(Wm ) sT 4 ;s 5.67 x108Wm 2 K 4 21 An Example 22 Interaction of Light and Matter States of Matter 23