* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Electron Excitement Notes
Survey
Document related concepts
Transcript
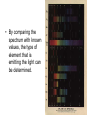
Electron Excitement Notes The Atom and Unanswered Questions Recall that in Rutherford's model, the atom’s mass is concentrated in the nucleus and electrons move around it. The model doesn’t explain how the electrons were arranged around the nucleus. The model doesn’t explain why negatively charged electrons aren’t pulled into the positively charged nucleus. The Atom and Unanswered Questions (cont.) In the early 1900s, scientists observed certain elements emitted visible light when heated in a flame. Analysis of the emitted light revealed that an element’s chemical behavior is related to the arrangement of the electrons in its atoms. The Wave Nature of Light Visible light is a type of electromagnetic radiation, a form of energy that exhibits wave-like behavior as it travels through space. All waves can be described by several characteristics. The Wave Nature of Light (cont.) The wavelength (λ) is the shortest distance between equivalent points on a continuous wave. The frequency (ν) is the number of waves that pass a given point per second. The amplitude is the wave’s height from the origin to a crest. The Wave Nature of Light (cont.) The Wave Nature of Light (cont.) The speed of light (3.00 108 m/s) is the product of it’s wavelength and frequency c = λν. The Wave Nature of Light (cont.) Light exists with varying amounts of frequencies (# of waves per second) and wavelengths (distance between each wave) Shorter wavelength/ Higher frequency corresponds to more energy, the opposite to less energy Sunlight contains a continuous range of wavelengths and frequencies. A prism separates sunlight into a continuous spectrum of colors. The electromagnetic spectrum includes all forms of electromagnetic radiation. The Wave Nature of Light (cont.) • --Visible light is light that has a wavelength within the range of 10-6 to 10-7 meters. If light has a longer wavelength, the color will be closer to red, and if light has a shorter wavelength, the color will be closer to violet. • R O Y G B IV • Light outside of the range of visible light cannot be detected by the human eye. • Generally any light with more energy than visible light is called Ultraviolet, and that with less energy is Infrared Atomic Emission Spectra Light in a neon sign is produced when electricity is passed through a tube filled with neon gas and excites the neon atoms. The excited atoms emit light to release energy. • When electrons are where they are predicted to be (from order in periodic table), they are considered to be in their ground state. • Sometimes atoms will absorb energy (ex. from electricity) and their electrons are given enough energy for them to “jump” to a higher energy level. This is considered an excited state. • Eventually they will lose the energy and fall back down to their ground state from the excited state. • When this falling occurs, the electrons emit the lost energy in the form of light. • When the electrons fall, they will release a color of light that will correspond to the amount of energy that is released from the fall. • The minimum amount of energy needed to make an electron jump is called a quantum. If an atom receives less than a quantum of energy, no light will be emitted • The light emitted will be in the form of a specific “particle” called a photon. It can be thought of as a “bundle” of energy • The longer the fall, the more energy is released, and the closer the color will be to violet. • If the fall is short, there is less energy released, and the color is closer to red. • Each element has a different number of electrons and therefore a different number of possible energy level changes. • Also, the spacing between orbitals varies with each element • Because of this, when each element is excited, the colors of light that will be released will vary. Atomic Emission Spectra (cont.) Atomic Emission Spectra (cont.) The atomic emission spectrum of an element is the set of frequencies of the electromagnetic waves emitted by the atoms of the element. Each element’s atomic emission spectrum is unique. • By comparing the spectrum with known values, the type of element that is emitting the light can be determined. • For instance, this shows four tubes containing different gases (neon, mercury, helium, and hydrogen) • A Spectroscope • It bends light to separate the colors that are found in within the light into different, distinct colors in different locations • When their light is shown through a spectroscope, different colors appear in different places for each different element