* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PYREXIA OF UNKNOWN ORIGIN
Cryptosporidiosis wikipedia , lookup
Typhoid fever wikipedia , lookup
Hepatitis C wikipedia , lookup
Marburg virus disease wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Oesophagostomum wikipedia , lookup
Hepatitis B wikipedia , lookup
Rocky Mountain spotted fever wikipedia , lookup
Neonatal infection wikipedia , lookup
Leptospirosis wikipedia , lookup
Schistosomiasis wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
Diagnosis of HIV/AIDS wikipedia , lookup
Epidemiology of HIV/AIDS wikipedia , lookup
Microbicides for sexually transmitted diseases wikipedia , lookup
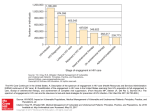
PYREXIA OF UNKNOWN ORIGIN Dr Henry Sunpath Head of Medicine Mc Cord Hospital 3 September 2009 Outline of presentation APPROACH TO PUO PUO AND HIV LOOKING FOR OTHER CAUSES=NTS POST ART =FEVER PRE ARTRE= AND FEVER MR B.S 28 years old Admitted 7/10/07 with Fever and Nt sweats > 3/52 found to have pericardial effusion on US scan and started emperically on TB tx also T/F 2 units of blood for normocytic anaemia Hb 5.6 Recent HIV test apparantly negative but result not seen by our team Given FU app for to assess response to tx Readmitted 16/10 PC persisting fevers and nt sweats with continued wt loss OE Pale, pyrexic small mobile LN lt posterior cervical area No rash, joints NAD, RS,ABDO NAD Soft PSM dynamic cardiac pulsation Pulse 104 BP 115/65 Neurological OE Normal Investigations FBC: Hb 7.38 Mcv 78 ESR 142 Wcc 18.6 Neut 15.7 Lym 2.19 Plats 557 Film : Hypochromic,microcytic UE: 131,3.6,102,26,3.6,74 LFT: NAD Alb 18 CXR: Normal Urinalysis NAD Working diagnosis 1: Bacterial endocardtis 2: TB with slow response to tx PLAN started on high dose penicillin and gentamicin awaiting blood cultures TB tx continued Sent for echo and US at Parklands ABDO US : Mild Splenomegaly otherwise NAD with no nodes seen ECHO : slightly dilated LV Good LV function , small pericardial effusion 0.8 mm No vegetations or valve lesions seen Blood culture Negative Continued investigatons Bone marrow: hypocellular marrow with no evidence of infiltration ,constistent with combined nutrional deficiency and systemic illness Malaria and widal negative WR negative C3 + C4 Normal awaiting ANF Lymph node histology =Follicular hyperplasia non specific finding Progress Completed 10/7 of iv AB and continued TB treament but spiking fever persisted Patient becoming increasingly despondant on ward and allowed to go home with FU Declined further HIV test while on ward despite counselling Problem list P.U.O Pericardial effusion Mild LV dilatation Mild splenomegaly Anaemia [microcytic]+hypocellular marrow Neutrophil leucocytosis Hypoalbuminaemia Possible Diagnoses Infective: Pulmonary/ Extrapulmonary TB Mycobacterium avium [MAI] MDR TB Disseminated fungal CMV Malignancy: Lymphoma Collagen vascular diseases Granulamatous : sarcoid Future plans on review ? CT SCAN of Chest and Abdomen looking for lymphoma or hidden abscess collection ? Try pericardial tap for culture and cytology ? Further biopsy ie Liver Repeated Blood cultures specifically looking for TB,Fungal inf etc Serological tests for CMV Any other ideas? [need to confirm HIV status] Fever of unknown origin Classic PUO investigation Nosocomial 3/52 Fever + 1 week of 3/7 OF IX Neutropenic 3/7 OF IX HIV asociated 4/52 OPD 3/7 inpatient CAUSES CLASSIC PUO INFECTIVE 20-30% CANCER 10-20% AUTOIMMUNE 15-20% MISC 15-25% UNDIAGNOSED 5-10% INFECTIVE Localised pyogenic infection Systemic bacterial eg typhoid Mycobacterial MTB,MAI Fungal eg cryptococcus Viral eg HIV,CMV Parastitic eg Malaria,Toxoplasmosis Rickettsial eg Q fever CANCERS LYMPHOMA LEUKAEMIA LIVER,RENAL,COLON,PANCREATIC SARCOMA ATRIAL MYXOMAS Collagen vascular diseases SLE RA PAN WEGENERS STILLS Polymyalgia rheumatica Rheumatic fever Behcets MISC GRANULOMATOUS DRUG ie Sarcoid ,Crohns induced fever ENDOCRINE ie thyrotoxicosis,phaeocrocytoma INTRACERBRAL ie SOL,pontine CVA,encephalitis METABOLIC /INHERITED ie familial mediteranian fever Tissue infarction ie Post MI, Rec PE HIV associated PUO HIV alone TB,M avium/intracelulare Toxoplasmosis CMV ,PCP ,Salmonella Cryptococcus,Histoplasmosis Non Hodgkins Lymphoma Drug induced OTHER CAUSES ASSOCIATED WITH FEVER=TTP/HUS Non Typhoid salmonella…the need to screen in SAVER=TTP/HUS, Look for candidemia as a cause of PUO in ICU pts- Nomenclature DNA hybridization studies show that medically important Salmonellae organisms can be considered as a single species known as Salmonellae enterica S. typhi S. paratyphi >2000 remaining Nontyphoid serotypes •Grouped as NTS Background- Salmonellae infection in HIV infected African adults Pattern of HIV related disease seen in Africa is known to be different from that seen in the developed world. (Grant AD, Djomand G, de Cock KM. Natural history and spectrum of disease in adults with HIV/AIDS in Africa. AIDS 1997;11(suppl B):S43-S54) NTS septicaemia is one of the most frequent manifestations of HIV in adults in Africa. Most case series have found that focal metastatic NTS infections in HIV are rare Bacterial infections and TB predominate Case reports of focal infection in literature Pulmonary involvement in HIV patients with NTS bacteraemia is well recognised May represent isolated NTS lung disease or co-infection with second respiratory pathogen Pattern of Bacteremia in HIV Positive African adults NTS as a common cause of bacteraemia illness in HIVpositive patients is now an established pattern in HIV endemic areas of Africa. (Grant AD, Djomand G, de Cock KM. Natural history and spectrum of disease in adults with HIV/AIDS in Africa. AIDS 1997;11(suppl B):S43-S54) This pattern is distinct from that seen in HIV-positive patients in Europe or North America. Greater proportion of total isolates are Gram-postive, and S. aureus is the most common organism • Related to either IV drug use or intravenous access devices Gram-negative isolates from smaller proportion of total • NTS are commonest group of organisms Clinical Course of NTS bacteraemia in HIV-Positive African Adults Non-typhoidal salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS 2002. Prospective study that enrolled 100 consecutive adult inpatients with NTS bacteraemia Patients Treated with chloramphenicol and survivors followed to detect recurrence. Microbiological findings NTS blood isolates from 100 cases 75 – S. typhimurium 19 – S. enteritidis 1 – S. typhimurium + S. enteritidis 5 – other Salmonella spp. Host Susceptibility for Salmonellosis Extremes of age Alteration of bowel endogenous bowel flora Diabetes Malignancy Rheumatological disorders Reticuloendothelial blockage From Malaria or sickle cell disease Therapeutic immunosuppression of all types HIV infection Anatomic disruptions Kidney stones, urinary tract abnormalities, gallstones, atherosclerotic endovascular lesions, schistosomiasis, and prosthetic devices may serve as foci for persistent Salmonella infection Focal NTS infection- Case Reports Focal infections due to non-typhi Salmonella in patients with AIDS: report of 10 cases and review. Clin Infec Dis. 1997 Sep; 25(3):690-7 Ten of 38 HIV-infected patients (26.3%) with salmonellosis documented over a period of 9 years had focal suppurative complications Infections of the urinary tract, lungs, and soft tissue, followed by arthritis, endocarditis, and meningitis were most frequently seen Infectious endocarditis due to non-typhi Salmonella in patients infected with human immunodeficiency virus: report of two cases and review. Clin Infect Dis. 1996 May;22(5):853-5 Focal NTS infection- Case Reports Salmonella pyomyositis in patients with the human immunodeficiency virus. Br J Rheumatol. 1995 Jun; 34(6):568-71 Salmonella septic arthritis in HIV patients. Br J Rheumatol. 1993 Jan;32(1):88 Nontyphoidal salmonella intracranial infections in HIV-infected patients. Clin Infec Dis 1997, 25:11181120. Non-typhi Salmonella adrenal abscess in an HIVinfected patient. Case Reports. Liver abscess due to Salmonella enteritidis in a returned travelor with HIV infection: case report and review of the literature. Rev. Inst. Med. Trop. S. Paulo. March-April, 2003;45(2): 115-117 Lung Involvement in HIV-positive Patients Salmonella Lung Involvement in Patients with HIV Infection. CHEST1997. Retrospective clinical study- studied records of all HIV-infected patients with Salmonella bacteraemia at a university tertiary hospital in Spain from Jan 87 to Dec 95. Findings Lung involvement was frequent Present in 35% of HIV positive patients with NTS bacteraemia 33% of those with lung involvement had definite Salmonella pulmonary infection Predisposing factors for focal disease were not apparent Focal lung involvement with NTS was not associated with worse prognosis 56% of those with lung involvement had Superinfection with other respiratory pathogen 28% PCP 17% pyogenic bacterial infections 11% TB Antimicrobial Susceptibility and Resistance In developed countries there is great concern over development of antimicrobial resistance Nairobi, Kenya 48%-56% of isolates were resistant to 3 or more of the routinely available antimicrobials Malawi Increase in fluroquinolone resistance among Salmonellae serotypes 5% resistant to chloramphenicol 73% resistant to co-trimoxazole 79% resistant to ampicillin 43% resistant to gentamycin 40% resistant to tetracycline Bangui in Central Africa (Kassa-Kelembho et al. Bacteremia in adults admitted to the Department of Medicine on Bangui Community Hospital (Central Africa Republic). Acta Tropica. 2003;89:67-72) 12% resistant to chloramphenicol 75% resistant to co-trimoxazole 80% resistant to amoxicillin 26% resistant to gentamycin 56% resistant to tetracycline 0% resistant to ceftriaxone 0% resistant to ciprofloxacin Conclusions Blood stream infections (BSI) with NTS are a major cause of morbidity and mortality in African patients with HIV. Treatment should be initiated empirically before final bacteriological results are available Knowledge about type of pathogens responsible for BSI and pattern of antibiotic resistance is key Conclusions Consider NTS in Pt with HIV, CD4 <200, or clinical evidence of HIV and p/w fever and GI symptoms, or respiratory involvement or fever of unknown origin Empirical treatment with chloramphenicol, ceftriaxone, ciprofloxacin appears appropriate High resistance to penicillins ARV treatment also prevents recurrence Sidovudine has been shown to protect against Salmonellae bacterial recurrence IRIS AND FEVER 35 yo M with HIV infection HPI: June 2006: Diagnosed HIV (+) in the setting of PCP. CD4 cell count: 9. July 28: Presented to begin ARVs. Pt complained of nausea and chronic diarrhea. Began D4T/3TC/EFV. Pt had an abnormal CXR thought to represent resolving PCP infection. Aug 10: Pt complained of nausea and vomiting and was treated with metoclopromide. Aug 21: Admitted with worsening nausea, vomiting and diarrhea. He noted loss of weight of 6 kg over 1 month. Unremarkable CXR (results not available). No AFB + sputum. Ultrasound showed 'splenic granulomas' but no abdominal lymphadenopathy. Pt was diagnosed with IRIS tuberculosis and he commenced standard combination antituberculosis therapy. Pt reportedly responded well clinically. Sept 12: Complained of persistent nausea and vomiting at outpatient visit. Oct 3: Admitted with delirium, fever & vomiting for 1 week Sodium 124 mmol/l; creatinine 80 mmol/l. Lumbar puncture: 16 lymphocytes/mm3, neutrophils 2/mm3, red blood cells 30/mm3. India Ink (+), gram stain (-). Protein 1.24 g/l, glucose 1.5 mmol/l. Cryptococcal CSF antigen (+). Diagnosed with IRIS cryptococcal meningitis Treated with amphotericin, continued antiretrovirals, cotrimoxazole and antituberculosis therapy. Became afebrile and mental status improved. Discharged on to chronic care facility to complete therapy. Received therapeutic lumbar punctures to manage intracranial pressure (never formally measured). Oct 20: Completed 14 days of amphotericin; changed to fluconazole 400 mg daily. Oct 24: Switched to AZT/3TC/EFV due to severe peripheral neuropathy. Pt remained at chronic care facility without fever but with "dull mental status," vomiting and hyponatremia. Thought to be clinically dehydrated and received intravenous fluids. Nov 2: Repeat CD4 5, VL unavailable. Nov 6: Clinically he failed to improved and developed neutropenia w/ decreased hematocrit of 23% but preserved platelets of 214 cells/ul. He died on Nov. 7 at chronic care facility. Differential diagnosis of worsening of symptoms after HAART therapy Patient with OI Treated with ART Asymptomatic immune recovery Return of original symptoms Relapse IRIS New Symptoms New OI Medication Side-effects IRIS Proposed Diagnostic Criteria for IRIS (Adapted from: ACTG 2006, Shelburne et al, 2002; French et Required Criteria al 2005) Worsening symptoms of Supportive Criteria inflammation/infection, Increase in CD4+ count of disease progression or >25 cells/オL enlargement of pre-existing Atypical presentation of lesions after definate clinical “opportunistic infections or improvement with antitumours” microbial therapy preHAART Biopsy demonstrating Temporal relationship with granulomatous starting antiretroviral therapy inflammation or unusually exuberant inflammatory Symptoms not explained by response newly acquired infection or disease, or the usual course Spontaneous resolution of a previously acquired without specific disease antimicrobial therapy Decrease in HIV RNA level by >1 log10 Immune Restoration OI Manifestations Are Often Atypical MAC Lymphadenitis CMV C. neoformans Pleocytosis Hepatitis C ALT Hepatitis B Focal Vitreitis, Uveitis Marked ↑HCV RNA & ↑HBV DNA CASE 1: M. TB Immune reconstitution 28 Black African female 24/40 gravid diagnosed with pulmonary tuberculosis and HIV positive in July 2002 Living with sister and 6 year old son recently in Zambia 3/12 contact with brother who had just died of TB Presentation • • • • • • 3/52 - SOB, cough, fevers, weight loss Febrile temp 40 0C Cachectic; Left upper lobe crepitations Baseline CD4 cell count 59 (11%) and HIV RNA viral load of 266,700 copies/ml Inadequate sputum sample; Bronchoscopy – BAL AFB negative (AFB culture positive) CXR – dense L hilum Many Pathogens and Syndromes Infectious Pathogens M. tuberculosis M. avium complex Cryptococcus Pneumocystis Cytomegalovirus Herpes simplex Histoplasmosis Hepatitis B and C • • • • • • • Auto-immune Herpes zoster PML (JC virus) Kaposi’s sarcoma (HHV8) M. leprae Bartonella Leishmania major Chlamydia trachomatis Adapted from French et al, AIDS 2004 • • • • Grave’s disease SLE Sarcoidosis Guillain Barre Epidemiology of IRIS (CID 2000;30:882) • Now >300 case series and reports in English literature 35 30 25 20 % 15 10 5 0 TB i p o s u c n c M e o c .x to M p y Cr AC V CM V B H V C H Immune Reconstitution Syndrome: Clinical Settings 1. “NEW” INFECTION CD4 <200, start ART MAC lymphadenitis 2. “EXISTING”INFECTION DX CMV retinitis start ART CMV immune vitriti FEVER BEFORE STARTING HAART History -34 yr old women tested HIV positive in Dec 2005. Presented in Jan 2006 to GP with oesophageal candidiasis and a history of cough with occasional loose stools since Nov 2005.She also had 8 kg wt loss over tst 6 months..No other OI or HIV related illness EXAMINATION T-38.5 WITH SINUS TACHYCARDIA,WASTING,PALLOR AND SEVERE ORAL TRUSH. NO LN,LIVER OR SPLEENIC ENLARGEMENT. RESPIRATORY EXAM =NORMAL INVESTIGATIONS Hb -6.9 g/dl NNA WCC 5.6 AND PLT -88 CD4-27 and VL=>750 000 S.Alb-22g/l LDH 3116 LFT otherwise normal Blood cultures –aerobic and anaerobic mycobacterial negative Bone marrow trephine and aspiratedisordered erytropoeisis in keeping with HIV disease,Histology of the trephine-no granulomas or signs of malignancy. The CXR was normal. Futhur course Treated with Diflucan and given blood transfusion. ART commenced Reg 1 a. Symptomatic peripheral peripheral neuropathy after 2 weeks-change to AZT. Four weeks later clinical deterioration with confusion and pallor but no fever. CXR=R pleural effusion-blood stained CYTOLOGY=high grade plasma blastic NON HODGKINS LYMPHOMA Refuse futhur treatment after assesment DISCUSSION Dillemma for clinicians of pt presenting with advanced HIV infection for the first time with prominent constitutional symptoms.Careful evaluation for Ois is unrevealing.Within weeks of nstarting ART a fatal disease process becomes apparent Advanced HIV infection can produce fever and wt loss /constitutional symptoms without underlying OI.However this is a diagnosis of exclusion These should be approached as PUO Various forms of TB are the most common causes of PUO.If cough is present then sputa should be sent for AFB stains. Other initial investigations should be FBC,WITH DIF COUNT LFT CRP URINE ANALYSIS-WBC CASTS ?culture CXR ULTRASOUND HEART/ABDOMEN to look for effusions/fluid,lymph nodes,hepatic annd splenic lesions and pelvic masses. TB IS SUGGESTED Bypulmonary infiltrates,fluid colections ,lymphnodes,splenic lesions, OTHER INFECTIONS AND MALIGNANCIES CAN ALSO PRESENT LIKE THIS! MYCOBACTERIAL BLOOD CULTIRES may be used to using the BACTEC/Myco /F lytic bottle TO DETECT BACTERIA,FUNGI ,NORCADIA as well as MTB and MAC and ?MOTT CSF studies –CRAG /India ink BMAT-if bicytopenia or pancytopenia present Liver biopsy if ALP and GGT raised. LN-FNAC or biopsy (normal saline and formalin) EMPERICAL TB TREATMENT WITH FOLLOW UP .SEND CULTURES AS FAR AS POSSIBLE OF POSSIBLE FOCAL SITE IF BY 8 WEEKS THERE IS NO OBJECTIVE RESPONSE-CONSIDER ALTERNATE DIAGNOSIS Conradie F;Wilson D ..SAHIVCS journal ,June 2006. References Chehimi J, Starr SE, Frank I et al. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J Exp Med 1994; 179:1361-1366 Casado JL, Navas E, Begona F, Moreno A, Martin P, Hermida JM, Guerrero A. Salmonella Lung Involvement in Patients with HIV Infection. Chest 1997, 112:1197-1201 Casado JL, Valdezate S, Calderon C, Navas E, Frutos B, Guerrero A, Martinez-Beltran J. Zidovudine Therapy Protects against Salmonella Bacteremia Recurrence in Human Immunodefieciency Virus- Infected Patients. J of Infec Dis 1999, 179: 1553-6 Gilks CF, Brindle RJ, Otieno LS, Simani PM, Newham RS, Bhatt SM, Lule GN, Okelo GB, Watkins WM, Waiyaki PG, et al. Life-threatening bacteremia in HIV-1 seropositive adults admitted to hospital in Nairobi, Kenya. Lancet 1990, 336(8714):545-9 Gordon MA, Banda HT, Gondwe M, Gordon SB, Boeree M, Walsh AL, Corkill JE, Hart CA, Gilks CF, Molyneux ME. Non-typhoidal salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS 2002, 16: 1633-1641 Gordon MA, Walsh AL, Chaponda M, Soko D, Mbvwinji M, Molyneux ME, Gordon SB. Bacteremia and Mortality Among Adult Medical Admissions in Malawi- Predominance of Non-typhi Salmonelllae and Streptococcus pneumonia. J of Infec 2001, 42: 44-49 Hart CA, Beeching NJ, Duerden BI. Infections in AIDS. J Med Microbiol 2000, Vol 49: 947-967 Hohmann EL. Nontyphoidal Salmonellosis. Clinical Infectious Disease 2001, 32:263-9 Kassa-Kelembho et al. Bacteremia in adults admitted to the Department of Medicine on Bangui Community Hospital (Central Africa Republic). Acta Tropica. 2003, 89:67-72 Vazquez-Torres A, Jones-Carson J, Baumler AJ et al. Extraintestinal dissemination of Salmonella by CD18-expressing phygocytes. Nature 1999; 401:804-808