* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Phenotypic diversity in acquired human prion diseases
Marburg virus disease wikipedia , lookup
Meningococcal disease wikipedia , lookup
Onchocerciasis wikipedia , lookup
Schistosomiasis wikipedia , lookup
Chagas disease wikipedia , lookup
Leptospirosis wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Neglected tropical diseases wikipedia , lookup
Visceral leishmaniasis wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Bovine spongiform encephalopathy wikipedia , lookup
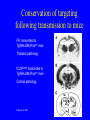
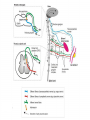
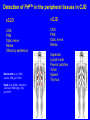
Overview • • • • • Nature of the infectious particle in TSE TSE strains Role of PrPC in disease Potential therapeutic targets Implications for other neurodegenerative diseases Prion Diseases: Transmissible Spongiform Encephalopathies • Fatal neurodegenerative diseases in man and mammals • Transmissible under natural and experimental conditions • Lengthy incubation period with no conventional host response • Characteristic neuropathology with spongiform change in grey matter • Associated with conversion of PrPC to PrPSc Prion diseases of humans and animals • Scrapie in sheep and goats • Transmissible mink encephalopathy • Chronic wasting disease in deer & elk • Bovine spongiform encephalopathy • Feline spongiform encephalopathy • Kuru • Creutzfeldt-Jakob disease • Gerstmann-StrausslerScheinker disease • Fatal familial insomnia • Variant CreutzfeldtJakob disease Protein-only version of the prion hypothesis • “Prions are transmissible particles that are devoid of nucleic acid and seem to be composed entirely of a modified protein (PrPSc).” • “The normal, cellular PrP (PrPC) is converted into PrPSc through a post-translational process during which it acquires a high beta-sheet content.” Prusiner SB, Proc Natl Acad Sci USA 1998;95:13363-83 Role of C PrP in TSE • PrPC is required for disease propagation and neuropathology • PrPC with GPI anchor to cell membrane transduces or potentiates the neurotoxicity of TSE infection • Tg PrP null mice do not propagate TSE infectivity • Tg mice expressing only anchorless PrPC can propagate TSE infectivity, but with greatly reduced neuropathology and clinical effects Infectious particle in prion diseases • Nonfibrillar particles between 300-600 kDa (mass equivalent to ~14-28 PrP molecules) • Other molecular constituents? • Cofactors for infectivity – sulphated GAG or nucleic acids? PrPres Isotype by Western blot Treatment with proteinase K results in N-terminal truncation of PrPres Distinct isotypes of PrPres characterize different forms of CJD Isotypes differ in extent of truncation and degree of glycosylation site occupancy Multiple conformations of Sc PrP ? • “In contrast to pathogens carrying a nucleic acid genome, prions appear to encipher strain-specific properties in the tertiary structure of PrPSc.” (Prusiner) • Is there evidence for heritable structural diversity in different prion diseases? PRNP codon 129 genotype frequencies MM MV VV Normal population 37% 51% 12% Sporadic CJD 71% 15% 14% vCJD 100% - - Idiopathic human prion diseases Prion disease Sporadic CJD (Myoclonic, Heidenhain variants) Sporadic CJD (Ataxic variant) Sporadic CJD (Kuru-plaque variant) Sporadic CJD (Sporadic fatal insomnia) Sporadic CJD (Cortical variant) Sporadic CJD PRNP mutation None PRNP codon 129 MM, MV PrPres Histological isotype correlate Type 1 None VV Type 2A None MV Type 2A None MM None MM None VV Type 2A Type 2A (Basic glycans) Type 1 Synaptic and coarse granular PrP staining in cortex. Plaque-like, focal and perineuronal PrP staining. Amyloid plaques in the cerebellum. Reference Parchi et al 1999 Parchi et al 1999 Parchi et al 1999 Thalamic atrophy. PrP staining faint and variable. Parchi et al 1999 Pan et al 2001 Cortical perivacuolar PrP staining. Parchi et al 1999 Pan et al 2001 Faint synaptic PrP staining. Parchi et al 1999 Do different PrPres types replicate with fidelity in vitro? When human PrPC is converted to PrPres in a PMCA reaction the product has both the conformation and the glycosylation ratio of the in-put PrPres Soto et al, 2005 Cellular co-factors & conversion: mammalian RNA Mammalian brain extracts contain RNA that stimulate the conversion of PrPC to PrPSc in a modified PMCA reaction (Deleault et al, Nature 2003;425:717-720) res PrP Conservation of isotype following transmission to mice PrPres (kDa) Inoculum Host Host PRNP None Human FFI(D178, M129) 19 FFI Mouse Tg(MHu2M) 19 FFI Tg(MHu2M) Mouse Tg(MHu2M) 19 None Human fCJD(E200K) 21 fCJD Mouse Tg(MHu2M) 21 fCJD Tg(MHu2M) Mouse Tg(MHu2M) 21 Telling et al 1996 Conservation of targeting following transmission to mice FFI transmitted to Tg(MHu2M)Prnp0/0 mice Thalamic pathology fCJDE200K transmitted to Tg(MHu2M)Prnp0/0 mice Cortical pathology Telling et al 1996 Aspects of PrPSc structure that might encipher strain properties • Extent of structural re-arrangement (conversion to b-sheet) at the N-terminus. • Presence of methionine or valine at codon 129 • Presence or absence of bound divalent cations (Cu2+) • Extent of of asparagine-linked glycosylation site occupancy • Composition and complexity of attached glycans Pathogenic mechanism • If we accept the centrality of of the conversion of PrPC to PrPSc in the pathogenic process, then there are in principle three possible alternatives: – The loss of an essential function of PrPC – The acquisition of a toxic function by PrPSc – Production of toxic intermediate or by-product Neurodegenerative mechanism Hope 2000 Problems with anti-TSE therapy • • • • Which compound(s) to use? What route of delivery to use? Is peripheral treatment required? How long to treat? Approaches to treatment of TSE • • • • Prevention of PrPC conversion Dissolution of PrPSc aggregates Enhanced PrPSc clearance Neuronal rescue? Strategies to prevent PrPC conversion • Inhibition of expression by RNA interference • Binding to site(s) for physiological ligands, resulting in PrPC clustering and internalisation from cell surface Compounds with in vivo antiTSE activity Class/compound Example Sulphonated dyes Sulphated glycans Cyclic tetrapyrroles Polyene antibiotics Quinolenes Metal chelators Tetracyclines Congo red pentosan polysulphate porphyrins amphotericin B quinacrine penicillamine doxycyline Detection of PrPSc in the peripheral tissues in CJD sCJD vCJD CNS PNS Optic nerve Retina Olfactory epithelium CNS PNS Optic nerve Retina Wadsworth et al, (2001), Lancet, 358, pp171-80 Head et al, (2004), American Journal of Pathology, 164, pp143-53 Appendix Lymph node Peyers’ patches Tonsil Spleen Thymus Probable pattern of tissue infectivity in vCJD INFECTIVITY CNS Infectivity (perhaps 1,000 times higher than LRS) LRS Infectivity TIME Onset of symptoms Neurodegenerative disease and aberrant protein deposition • Classical neuropathology identifies abnormal histological structures which are diagnostic for particular conditions. • Nuclear and cytoplasmic inclusion bodies and extracellular amyloid deposits • Proteinaceous, fibrillar, and rich in b-pleated sheet secondary structure • “Fatal attractions” between abnormally folded forms of specific normal cellular proteins resulting in specific neurodegenerative diseases • A common feature of Alzheimer disease, Parkinson disease, Huntington disease, amyotrophic lateral sclerosis and prion diseases Neurodegenerative diseases associated with abnormal protein conformations (toxic gain of function) Disease Gene product Alzheimer’s disease Creutzfeldt-Jakob disease Parkinson’s disease Huntingdon’s disease Machado-Joseph disease (SCA 3) APP and Ab PrPc and PrPSc a synuclein Huntingtin Ataxin 3 Neuronal vulnerability to “toxic gain of function” • Neurones are post-mitotic cells which cannot be replaced (liable to damage by increasing DNA mutations?) • Unique metabolic demands - some neurones have to maintain an axon over 1m in length • Functional plasticity • Environment subject to control by many other structures, including astrocytes and the blood-brain barrier Review • • • • • Nature of the infectious particle in TSE TSE strains Role of PrPC in disease Potential therapeutic targets Implications for other neurodegenerative diseases