* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Congenital Heart Defects
Cardiovascular disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Myocardial infarction wikipedia , lookup
Cardiac surgery wikipedia , lookup
Coronary artery disease wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Atrial septal defect wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
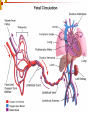
CHD ASD PDA VSD Tetrology of Fallot Tricuspid Artesia Transposition of great vessels Fetal Circulation Oxygenated blood from Umb. Vein bypasses liver and joins deoxygenated blood via DV. > Then it joins SVC and empties in Rt atrium. > through PFO it enters LA. Blood enters aorta through DA and bypasses pulm. Trunk Some blood goes to RV but through DA it again enters aorta to bypass pulm. Trunk. Deoxygenated blood returns via umbilical arteries.. 1. 2. 3. 4. Postnatal transition With first breath, Increased Alv. Pressure causes vasodilation in pul. Vessels. Obs. Clamping induces constriction and changes UV to Ligamentum teres and UA to Medial umbilical ligaments. DA becomes Ligamentum arteriosum ( 10-15 minutes) Foramen ovale closes and becomes fossa ovalis Causes of central cyanosis in the neonate Right-to-left shunt 1) Intracardiac level: cyanotic disease, anomalous systemic venous connection to left atrium 2) Great vessel level: persistent pulmonary hypertension of the newborn 3) Intrapulmonary level: pulmonary arteriovenous malformation Ventilation/perfusion mismatch 1) Airway disease: pneumonia, aspiration, cystic adenomatoid malformation, diaphragmatic hernia, pulmonary hypoplasia, labor emphysema, atelectasis, pulmonary hemorrhage, hyaline membrane disease, transient tachypnea of the newborn 2) Extrinsic compression of lungs: pneumothorax, pleural effusion, chylothorax, hemothorax, thoracic dystrophy Alveolar hypoventilation Central nervous system depression: asphyxia, maternal sedation, intraventricular hemorrhage, seizure, meningitis, encephalitis Neuromuscular disease: Werdnig-Hoffman disease, neonatal myasthenia gravis, phrenic nerve injury Airway obstruction: choanal atresia, laryngotracheomalacia, macroglossia, Pierre Robin syndrome Hemoglobinopathy: Methemoglobinemia: congenital or secondary to toxic exposure Other hemoglobinopathies Diffusion impairment Pulmonary edema: left-sided obstructive cardiac disease, cardiomyopathy, Pulmonary fibrosis, Congenital lymphangiectasia More than 32,000 infants (one out of every 125 to 150) are born with heart defects each year in the United States 1% of newborn affected by CHD The development of the cardiovascular system … Begins to develop toward the end of the third week The critical period of heart development is from day 20 to day 50 after fertilization. Three shunts in the fetal circulation 1)Ductus arteriosus 2) Ductus Venosus 3) Foramen Ovale Signs and symptoms suspicious for neonatal congenital heart disease Poor feeding: bottle feedings longer than 20 to 30 minutes, taking too little volume, resting frequently during feeds, or otherwise unexplained choking, gagging and/or frequent vomiting with feeds Breathing too fast or hard, particularly increasing with feeds Persistent unexplained cough or wheeze Color changes: central cyanosis, persistent pallor, grey, Excessive sweating, even while sleeping, increasing with feeds and other exertion Excessive, unexplained irritability Decreased activity; increased or excessive sleepingPoor weight gain 1. 2. 3. 4. 5. Most frequent Congenital heart anomalies Patent Ductus arterious Transposition of great vessels Hypoplastic left heart syndrome Tetrology of Fallots Pulmonary atresia Classification Cyanotic CHD Infants unable to achieve PaO2 of 100 % after inspiring of 100 % O2. Cyanosis Murmur CXR and ECG If PaO2 is low start PGE1 infusion. TOF, TA, Transposition, Truncus Arteriorus, TAPVR,HLHS Acyanotic CHD Infants will able to achieve PaO2 of > 100 % in same condition Murmur Symptoms of CHF Treatment vary from observation, PGE1 infusion to treatment of arrythmia ASD, VSD, PDA, HLHS, Coarc of aorta, av canal Transposition of great vessles Most common, M:F> 2: 1 P/E CXR- Egg on stick appearance Echo Catheterization- diagnostic and often therapeutic Treatment: if sever hypoxia of acidosis occurs, urgent atrial balloon septostomy and subsequent arterial switch operation is done Tetrology of Fallots ( PS, RVH, Overriding aorta, VSD) Male predominance P/E CXR- boot shaped heart with decreased pul. Vascular markings. EKG Echo-diagnostic Tt: If Pul. Flow is ductal dependent will benefit from PGE1 infusion Acyanotic Heart Diseases 100 % O2 test Murmur Signs of CHF VSD, ASD, PDA, HLHS VSD Most common Spontaneous closure in Half of the patients M:F > 1:1 Takes upto 4 wks to develop CHF ASD Mostly closes spontaneously May develop CHF but not in neonatal period PDA Large vessel connects pul. Trunk with descending aorta. Persistent patency of fetal channel after birth Closure in utero may lead to fetal demise of PHT Factors associated with PDA 1. 2. Associated with Increased incidence Prematurity 45 % in BW < 1750 gms 80 % in BW < 1000 gms RDS and surfactant treatment Fluid administration: Increased iv fluid load in first few days of life > increased incidence of PDA Asphyxia Congenital syndromes High altitude As a part of CHD 1. 2. 3. Associated with Decreased incidence Antenatal steroid administration IUGR PROM Clinical signs and presentation of PDA Murmur Bounding pulses and increased pulse pressure Hypotension Respi deterioration Diagnosis and Mx Diagnosis Echo Radiologic signs Mx Ventilatory support Fluid Restriction Increase HCt Indomethacin Ibuprofen Surgery Cx and C/I of Indomethacin Cx Renal Defects( transient decrease in GFR) GI bleeding Platelet function C/I of indomethacin S. Creatinine> 1.7 Frank GI or renal Bleeding NEC Sepsis Arterial blood gas • A blood gas measurement on an arterial sample should be obtained in any newborn with cyanosis. •An arterial PO2 value provides more specific data than oxygen saturation. •Because of the increased affinity of fetal hemoglobin for oxygen, PO2 values at a given level of oxygen saturation are often lower in newborns than adults. •An elevated arterial PCO2 value often indicates the presence of pulmonary disease. PCO2 may also be increased in heart failure. •A reduced pH level raises concern about poor cardiac output. The combination of severe hypoxemia, metabolic acidosis, and marked hypercarbia may occur in patients with d-transposition of the great arteries when there is inadequate mixing at the levels of the atria, ventricles, and great vessels. •Patients with methemoglobinemia typically have low oxygen saturation and normal oxygen tension., the blood has a chocolate-brown color Hyperoxia test The hyperoxia test is useful in distinguishing cardiac from pulmonary causes of cyanosis. In CHD associated with intracardiac right-to-left shunting, blood in the pulmonary veins is fully saturated with oxygen in ambient air. Administering higher concentrations of inspired oxygen increases the amount of dissolved oxygen but has minimal effect on oxygen tension levels. In contrast, patients with pulmonary disease have pulmonary venous desaturation. Supplemental oxygen administration in pulmonary disease typically increases pulmonary venous oxygen levels and improves systemic oxygenation. The preductal oxygen tension while breathing 100 percent oxygen concentration rarely exceeds 150 mmHg in cyanotic heart disease, and usually exceeds this value in pulmonary disease Mx General approach — The initial approach includes cardiorespiratory support and monitoring. If there is respiratory compromise, an adequate airway should be established immediately and supportive therapy (eg, oxygen, mechanical ventilation) instituted as needed. Patients with hypotension or poor perfusion require cardiopulmonary resuscitation. Antibiotics — Sepsis can lead to cyanosis and left ventricular dysfunction or pulmonary disease. As a result, unless another specific etiology is promptly identified, broad spectrum antibiotics should be initiated (ampicillin and gentamicin) after obtaining a complete blood count, urinalysis, and blood and urine cultures. Prostaglandin E1 — An infant who fails the hyperoxia test and does not have persistent pulmonary hypertension of the newborn or a chest radiograph consistent with lung disease is likely to have a congenital heart defect that is dependent upon the ductus arteriosus for pulmonary or systemic blood flow. If metabolic acidosis is present or if timely echocardiography is not available, prostaglandin E1 should be administered until a definitive diagnosis is established. This is usually started as an intravenous infusion in a dose of 0.05 µg/kg per minute DEFINITION — Cyanosis is a bluish discoloration of the tissues that results when the absolute level of reduced hemoglobin in the capillary bed exceeds 3 g/dL [6-8]. The appearance of cyanosis depends upon the total amount of reduced hemoglobin rather than the ratio of reduced to oxygenated hemoglobin. Two mechanisms result in an increased concentration of reduced hemoglobin in the capillary bed that in turn leads to cyanosis: increased oxygen extraction by the tissues and systemic arterial oxygen desaturation. Based upon these mechanisms, two types of cyanosis are described, peripheral and central. Central cyanosis — Central cyanosis, the focus of this topic review, is a pathologic condition caused by reduced arterial oxygen saturation. It involves highly vascularized tissues, such as the lips and mucous membranes, through which blood flow is brisk and the arteriovenous difference is minimal. Cardiac output typically is normal, and patients have warm extremities. Peripheral cyanosis — Patients with peripheral cyanosis have a normal systemic arterial oxygen saturation and increased oxygen extraction, resulting in a wide systemic arteriovenous oxygen difference. The increased extraction of oxygen results from sluggish movement of blood through the capillary circulation. Causes include vasomotor instability, vasoconstriction caused by exposure to cold, venous obstruction, elevated venous pressure, polycythemia, and low cardiac output. Peripheral cyanosis affects the distal extremities and circumoral or periorbital areas [6]. The extremities are often cool or clammy. Peripheral cyanosis may be associated with sepsis but is also seen in normal newborns, especially those with fair complexions. ETIOLOGY — Hypoxemia, with decreased arterial oxygen saturation leading to central cyanosis, results from the following mechanisms (show table 1) [6]: Right-to-left shunting at the intracardiac, great vessel, or intrapulmonary level Ventilation-perfusion mismatch Alveolar hypoventilation Hemoglobinopathy (including methemoglobinemia) that limits oxygen transport (show figure 3) [11] Diffusion impairment Noncardiac causes — Many noncardiac abnormalities can lead to cyanosis. (See "Suspected heart disease in the newborn: Criteria for referral"). Pulmonary disorders are the most common causes and include structural abnormalities of the lung, ventilation-perfusion mismatching, congenital or acquired airway obstruction, pneumothorax, and hypoventilation. Abnormal forms of hemoglobin (eg, methemoglobin, show table 2) can result in cyanosis, and polycythemic infants may appear cyanotic even if they are adequately oxygenated. (See "Genetic disorders of hemoglobin oxygen affinity" and see "Neonatal polycythemia"). Poor peripheral perfusion may result from sepsis, hypoglycemia, dehydration, and hypoadrenalism. Right-to-left shunting through the ductus arteriosus, resulting in a differential between SaO2 measured in the arm (preductal) and leg (postductal), can occur with primary or persistent pulmonary hypertension Cardiac causes — Cardiac causes of central cyanosis have been classified using different systems A frequently used mnemonic is the "five Ts" of cyanotic CHD: Transposition of the great arteries Tetralogy of Fallot Truncus arteriosus Total anomalous pulmonary venous connection Tricuspid valve abnormalities. A sixth "T" is often added for "tons" of other diseases, such as double outlet right ventricle, pulmonary atresia, multiple variations of single ventricle, hypoplastic left heart syndrome, complex conditions associated with heterotaxy syndromes, or anomalous systemic venous connection (left superior vena cava connected to the left atrium). We find that a more useful classification of cyanosis separates the defects into physiologic categories based upon decreased pulmonary blood flow, increased pulmonary blood flow, or severe heart failure. Autosomal dominant Albright hereditary osteodystrophyCardiomyopathy Ehlers-DanlosRupture of large vessels Holt-OramASD, VSD LeopardPS, prolonged PR interval MarfanAortic aneurysm, AI, MVP Myotonic dystrophyCardiomyopathy NeurofibromatosisCOA, renal artery stenosis Osler-Weber-RenduMultiple telangiectasis, pulmonary AVF Treacher CollinsASD, VSD, PDA Tuberous sclerosisMyocardial rhabdomyoma, WPW NoonanPS, ASD, AS, subaortic stenosis Autosomal recessive inheritance CarpenterPDA CockayneAtherosclerosis Cutis laxaPulmonary hypertension Cystic fibrosisCor pulmonale Ellis-van CreveldASDFriedreich ataxiaCardiomyopathy HomocystinuriaThromboses MPS type I H (Hurler)Coronary artery disease, AI, MIMPS type I S (Scheie)Aortic valve disease MPS type IV (Morquio)Aortic valve disease MPS type VI (Maroteaux-LamyAortic valve diseasePompe disease (acid maltase deficiency, GSD type 2)Cardiomyopathy Pseudoxanthoma elasticumCoronary artery disease, MIRefsum diseaseArrhythmiaSmith-Lemli-OpitzVSD,PDAThrombocytopenia absent radii (TAR)ASD, TOF X-linked inheritence (Hunter)Coronary artery disease, valvular diseaseDuchenne muscular dystrophyCardiomyopathyEmery-Dreifuss muscular dystrophy CardiomyopathyIncontinentia pigmentiPDA, hypertensionFabry diseaseCoronary artery disease