* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Organic Chemistry: Introduction
Survey
Document related concepts
Transcript
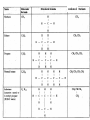
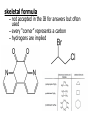
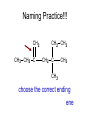
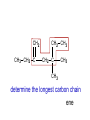
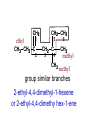
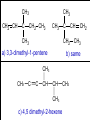
Organic Chemistry Topic 10.1.1 – 10.1.8 1 2 3 4 bonds HONC What is organic chemistry? • study of carbon, the compounds it makes, and the reactions it undergoes • over 16 million carbon-containing compounds are known • because the C-C single bond (348 kJ mol-1) and the C-H bond (412 kJ mol-1) are strong, carbon compounds are stable • carbon can form chains and rings Homologous series/compounds (10.1.1) • related compounds that have the same functional group (groups of atoms found within molecules that are involved in the chemical reactions characteristic of those molecules) • differ from each other by a CH2 unit • can be represented by a general formula – examples: • CnH2n+2 (alkanes) or CnH2n (alkenes) or… • have similar chemical properties • have physical properties that vary in a regular manner as the number of carbon atoms present increases – Example: the alkanes Trends in boiling points of members of a homologous series (10.1.2) • melting point and boiling point increase Alkane Formula Boiling Pt./oC with more carbon atoms methane CH4 -162.0 • Why? – – intermolecular forces increase adding a CH2 adds more electrons • this increases the Van der Waal’s forces ethane C2H6 -88.6 propane C3H8 -42.2 butane C4H10 -0.5 Empirical, molecular & structural formulas (10.1.3) • empirical formula – simplest ratio of atoms in a molecule • molecular formula – actual numbers of atoms in a molecule Empirical Formula Molecular Formula CH4 CH4 CH3 C2H6 CH2O C6H12O6 CH2 C4H8 CH2 C8H16 structural formula • unambiguously shows how the atoms are bonded together • can use condensed structural formulas – bonds are omitted, repeated groups put together, side chains put in brackets • CH3CH2CH2CH2CH2CH3 –or even CH3(CH2)4CH3 • CH3CH(CH3)CH3 (this is not the molecule above) condensed skeletal formula – not accepted in the IB for answers but often used – every “corner” represents a carbon – hydrogens are implied Isomers (10.1.4) • (structural) isomers: compounds with the same molecular formula but different structure (arrangement of atoms) • different isomers are completely different compounds • have different physical properties such as melting point and boiling point Structural Formulas for C4H10O Isomers Alkanes Structural formulas for the isomers of noncyclic alkanes up to C6 (10.1.5) • hydrocarbon chains where all the bonds between carbons are SINGLE bonds • CnH2n+2 • draw out and write the structural formulas for all isomers that can be formed by: – – – – – – CH4 C2H6 C3H8 C4H10 C5H12 C6H14 Richard Thornley 10.1.5 2:54 Naming the isomers (IUPAC) of non-cyclic alkanes up to C6 (10.1.6) 1. Richard Thornley 3:35 2. Determine the longest carbon chain – 1 2 3 4 5 6 Use the prefix to denote the number carbons MethEthPropButPentHex- Monkeys Eat Peeled Bananas 3. use the suffix “-ane” to indicate that the substance is an alkane 4. number the carbons in the chain consecutively, starting at the end closest to a substituent (groups attached to the main chain/most busy end) 5. name and number the location of each substituent – the name of the substituent will be written before the main chain and will end with “–yl” (or just memorize the below) • • • CH3 is methyl C2H5 is ethyl C3H7 is propyl And with 2 or more side chains: 5. use prefixes di-, tri-, tetra-, to indicate when there are multiple side chains of the same type 6. use commas to separate numbers and hyphens to separate numbers or letters. 7. name the side chains in alphabetical order • How about C5H12? The isomers are: Pentane 2-methyl-butane dimethyl-propane Alkenes Structural formulas for the isomers of the straight chain alkenes up to C6 (10.1.7) • alkenes have a double bond between two or more of the carbons • CnH2n • draw out and write the structural formulas for all isomers that can be formed by each – C2H4 – C3H6 Richard Thornley 10.1.7 (1:37) – C4H8 – C5H10 – C6H12 Nomenclature Practice Name this compound CH3 H3C1 2 Cl 3 4 5 CH3 9 carbons = nonane 6 7 8 H3C9 Step #1: For a branched hydrocarbon, the longest continuous chain of carbon atoms gives the root name for the hydrocarbon Nomenclature Practice Name this compound CH3 H3C1 2 Cl 3 4 5 CH3 6 9 carbons = nonane CH3 = methyl 7 8 chlorine = chloro H3C9 Step #2: When alkane groups appear as substituents, they are named by dropping the -ane and adding -yl. Nomenclature Practice Name this compound CH3 H3C1 2 Cl 3 4 5 9 carbons = nonane CH3 6 CH3 = methyl 7 chlorine = chloro 8 H3C9 1 9 NOT 9 1 Step #3: The positions of substituent groups are specified by numbering the longest chain of carbon atoms sequentially, starting at the end closest to the branching. Nomenclature Practice Name this compound CH3 H3C1 2 Cl 3 4 5 CH3 9 carbons = nonane 6 CH3 = methyl 7 8 chlorine = chloro H3C9 2-chloro-3,6-dimethylnonane Step #4: The location and name of each substituent are followed by the root alkane name. The substituents are listed in alphabetical order (irrespective of any prefix), and the prefixes di-, tri-, etc. are used to indicate multiple identical substituents. Naming the isomers (IUPAC) of straight chain alkenes up to C6 (10.1.8) 1. suffix changes to “-ene” 2. when there are 4 or more carbon atoms in a chain, the location of the double bond is indicated by a number 3. begin counting the carbons closest to the end with the C=C bond – numbering the location of the double bond(s) takes precedence over the location of any substituents 1-butene but-1-ene 2-butene but-2-ene Naming Practice!!! CH2 CH3 CH2 C CH2 CH3 CH2 C CH3 CH3 choose the correct ending ene CH2 CH3 CH2 C CH2 CH3 CH2 C CH3 CH3 determine the longest carbon chain ene CH2 CH3 CH2 C CH2 CH3 CH2 C CH3 CH3 assign numbers to each carbon ene CH2 CH2 CH3 1 CH3 CH2 C 2 5 CH2 C 3 4 6 CH3 CH3 assign numbers to each carbon ene CH2 CH2 CH3 1 CH3 CH2 C 2 5 CH2 C 3 4 6 CH3 CH3 attach prefix (according to # of carbons) 1-hexene ene CH2 ethyl CH2 CH3 1 CH3 CH2 C 2 5 CH2 C 3 4 CH3 6 CH3 methyl methyl determine name for side chains 1-hexene CH2 ethyl CH2 CH3 1 CH3 CH2 C 2 5 CH2 C 3 4 CH3 6 CH3 methyl methyl attach name of branches alphabetically 2-ethyl-4-methyl-4-methyl-1-hexene 1-hexene CH2 ethyl CH2 CH3 1 CH3 CH2 C 2 5 CH2 C 3 4 CH3 6 CH3 methyl methyl group similar branches 2-ethyl-4-methyl-4-methyl-1-hexene 1-hexene CH2 ethyl CH2 CH3 1 CH3 CH2 C 2 5 CH2 C 3 4 CH3 6 CH3 methyl methyl group similar branches 2-ethyl-4,4-dimethyl-1-hexene or 2-ethyl-4,4-dimethy hex-1-ene CH3 CH CH2 propene CH3 CH CH3 CH3 CH CH3 CH C CH3 CH CH3 2-butene 2,4-dimethyl-2-pentene 2,4-dimethyl pent-2-tene CH3 CH2 CH C CH3 CH2 CH3 CH3 CH3 C CH CH2 a) 3,3-dimethyl-1-pentene C C CH CH3 b) same CH3 CH3 CH2 CH CH3 c) 4,5 dimethyl-2-hexene CH3