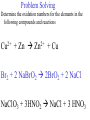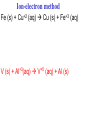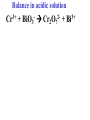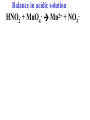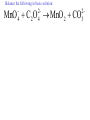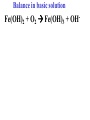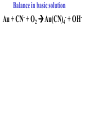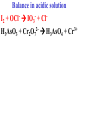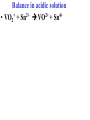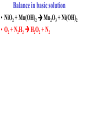* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ch5_f08
History of electrochemistry wikipedia , lookup
Surface properties of transition metal oxides wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Ionic compound wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Electrolysis of water wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Oxidation-Reduction Reactions Fe2O3(s) + 2 Al(s) 2 Fe(s) + Al2O3(s) Google Video: Thermite Rxn Chapter 6: Oxidation-Reduction Reactions Reactions that involve the transfer of electrons are called oxidation-reduction or redox reactions OIL RIG or LEO says GER • Oxidation is the loss of electrons by a reactant • Reduction is the gain of electrons by a reactant • oxidizing agent • reduction agent Oxidation numbers provide a way to keep track of electron transfers : 1) The oxidation number of any free element is zero. 2) The oxidation number of any simple, monoatomic ion is equal to the charge on the ion. 3) The sum of all oxidation numbers of the atoms in a molecule or polyatomic ion must equal the charge on the particle. 4) In its compounds, fluorine has an oxidation number of –1. 5) In its compounds, hydrogen has an oxidation number of +1. 6) In its compounds, oxygen has an oxidation number of –2. p.218 Problem Solving Determine the oxidation numbers for the elements in the following compounds and reactions MoO2 BrO3- KN3 HPO4Zr(SrO4)2 Mg2TiO4 Fe2O3 Question: What is the oxidation number of P in H3PO4? a: -5 b: -3 c: 0 d: +3 e: +5 Problem Solving Determine the oxidation numbers for the elements in the following compounds and reactions Cu2+ + Zn Zn2+ + Cu Br2 + 2 NaBrO2 2BrO2 + 2 NaCl NaClO3 + 3HNO2 NaCl + 3 HNO3 Question: The oxidation number of an atom increases during a reaction. This means that the atom was____________ and the substance containing it was the____________ a: oxidized; reducing agent b: reduced; oxidizing agent c: oxidized; oxidizing agent d: reduced; reducing agent Question: In a redox titration, MnO4- reacts to Mn2+. In this reaction, is Mn reduced or oxidized? a: reduced b: oxidized Question: What is the reducing agent in this reaction? Sn + Cl2 SnCl2 a: Cl2 b: Sn c: SnCl2 2 3 Al( s) Cu (aq) Al (aq) Cu( s) • The oxidation and reduction are divided into equations called halfreactions • The half-reactions are balanced separately, then combined into the fully balanced net ionic equation • Both mass and charge must be balanced • Charge is balanced by adding electrons to the side of the equation that is more positive or less negative Al( s ) Cu 2 (aq) Al 3 (aq) Cu( s ) ANALYSIS : This is a redox reaction. SOLUTION : Oxidation : Al( s ) Al 3 3e Reduction : Cu 2 (aq) 2e - Cu( s) The least common factor is 6, combining 2 Al( s ) 3Cu 2 (aq) 2Al 3 (aq) 3Cu( s ) Ion-electron method Fe (s) + Cu+2 (aq) Cu (s) + Fe+3 (aq) V (s) + Al+3(aq) V+5 (aq) + Al (s) Question: What is the coefficient of Ce3+ when the equation is balanced with the ion-electron method? 3+ Ce + a: 1 b: 2 c: 3 d: 4 e: 5 5+ V 2+ V + 4+ Ce The Ion-Electron Method in Acidic Solution: 1) 2) 3) 4) 5) 6) Divide the equation into two half-reactions. Balance atoms other than H and O. Balance O by adding water. Balance H by adding hydrogen ion. Balance net charge by adding electrons. Make electron gain and loss equal: add halfreactions. 7) Cancel anything that’s the same on both sides of the equation. p. 227 Balance in acidic solution 3+ Cr + BiO3 Cr2O7 + - 2- 3+ Bi Balance in acidic solution HNO2 + MnO4- Mn2+ + NO3- Question: What must always cancel in writing the net balanced redox equation? a: H+ b: H2O c: OHd: e- Additional Steps for Basic Solutions 8) Add to both sides of the equation t he same number of OH- ions as there are H . 9) Combine H and OH- to form H 2O. 10) Cancel any H 2 O that you can. Balance the following in basic solution: MnO C 2 O MnO 2 CO 4 24 23 MnO -4 C 2 O 24- MnO 2 CO32ANALYSIS : Balance as if in acidic solution t hen " convert". SOLUTION : C 2 O 24- 2H 2 O 2CO32- 4H 2e MnO -4 4H 3e - MnO 2 2H 2 O Net ionic : 3C 2 O 24- 2H 2 O 2MnO -4 6CO32 4H 2MnO 2 Add OH3C 2 O 24- 2H 2 O 4OH- 2MnO -4 6CO32 4(H OH ) 2MnO 2 Form H 2 O 3C 2 O 24- 2H 2 O 4OH- 2MnO -4 6CO32 4H 2 O 2MnO 2 Simplify 3C 2 O 24- 4OH- 2MnO -4 6CO32 2H 2 O 2MnO 2 Balance in basic solution Fe(OH)2 + O2 Fe(OH)3 + OH Balance in basic solution Au + CN + O2 Au(CN)4 + - OH What is the coefficient indicated when the following is balanced? MnO4-(aq) + Cr (s) → Cr2O72-(aq) + ? MnO2(s) A. 1 B. 2 C. 3 D. 4 E. none of these 23 What is the coefficient indicated when the following is balanced? ? PbSO4(s) →Pb(s) + PbO2(s) + H2SO4(aq) A. 1 B. 2 C. 3 D. 4 E. none of these 5.2 The ion–electron method creates balanced net ionic equations for redox reactions 24 Reactions of metals with acids Zn + H2SO4 ZnSO4 + H2 Cu + H2SO4 no reaction Zinc is more active than hydrogen Copper is less active than hydrogen The more active will be Nonoxidizi ng acids : HCl( aq ), cold dilute H 2SO 4 (aq ), H 3 PO 4 (aq ), and most organic acids. Ox. Acid HNO 3 Reduction Reaction (conc.) NO3- 2H e - NO2 ( g ) H 2 O (dilute) NO3- 4H 3e - NO(g ) 4H 2 O (very dilute, with strong reducing agent) 4 NO 10H 8e NH 3H 2 O 3 H 2SO 4 - (hot, conc.) SO 24- 4H 3e - SO 2 ( g ) 2H 2 O (hot conc., with strong reducing agent) SO -4 10H 8e - H 2S( g ) 4H 2 O Table 6.1 p231 Nitric acid acts upon copper • Ira Remsen (1846-1927) founded the chemistry department at Johns Hopkins University, and founded one of the first centers for chemical research in the United States; saccharin was discovered in his research lab in 1879. Ira Remsen (1846-1927) founded the chemistry department at Johns Hopkins University, and founded one of the first centers for chemical research in the United States; saccharin was discovered in his research lab in 1879. Like many chemists, he had a vivid "learning experience," which led to a heightened interest in laboratory work: While reading a textbook of chemistry I came upon the statement, "nitric acid acts upon copper." I was getting tired of reading such absurd stuff and I was determined to see what this meant. Copper was more or less familiar to me, for copper cents were then in use. I had seen a bottle marked nitric acid on a table in the doctor's office where I was then "doing time." I did not know its peculiarities, but the spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words "act upon" meant. The statement "nitric acid acts upon copper" would be something more than mere words. All was still. In the interest of knowledge I was even willing to sacrifice one of the few copper cents then in my possession. I put one of them on the table, opened the bottle marked nitric acid, poured some of the liquid on the copper and prepared to make an observation. But what was this wonderful thing which I beheld? The cent was already changed and it was no small change either. A green-blue liquid foamed and fumed over the cent and over the table. The air in the neighborhood of the performance became colored dark red. A great colored cloud arose. This was disagreeable and suffocating. How should I stop this? I tried to get rid of the objectionable mess by picking it up and throwing it out of the window. I learned another fact. Nitric acid not only acts upon copper, but it acts upon fingers. The pain led to another unpremeditated experiment. I drew my fingers across my trousers and another fact was discovered. Nitric acid acts upon trousers. Taking everything into consideration, that was the most impressive experiment and relatively probably the most costly experiment I have ever performed. . . . It was a revelation to me. It resulted in a desire on my part to learn more about that remarkable kind of action. Plainly, the only way to learn about it was to see its results, to experiment, to work in a laboratory. from F. H. Getman, "The Life of Ira Remsen"; Journal of Chemical Education: Easton, Pennsylvania, 1940; pp 9-10; quoted in Richard W. Ramette, "Exocharmic Reactions" in Bassam Z. Shakhashiri, Chemical Demonstrations: A Handbook for Teachers of Chemistry, Volume 1. Madison: The University of Wisconsin Press, 1983, p. xiv: An Active Metal One metal can displace another from solution Zn (s) + CuSO4 (aq) Cu (s) + ZnSO4 (aq) Zinc is “more active” than copper Zinc is more active than copper • The more active metal will displace the lesser active metal from solution An activity series arranges metals according to their ease of oxidation • They can be used to predict reactions Copper is less active than Zinc Solid copper placed into zinc nitrate solution Activity Series for Some Metals and Hydrogen Element Oxidation Product Least active Gold Au 3 Silver Ag Copper Cu 2 Hydrogen H Most active Sodium Na Cesium Cs A given element will be displaced from its compounds by any element below it in the table (See Table 6.2 for a more extensive list.) Reactivity with acid Using the following observations, rank these metals from most reactive to least reactive • Cu(s) + HCl(aq) → no reaction • Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g) • Mg(s) + ZnCl2(aq) → MgCl2(aq) + Zn(s) a. Mg > Zn > H > Cu b. Mg > H > Cu > Zn c. Cu > H > Zn > Mg d. H > Cu > Mg > Zn 35 Activity Series 5.4 A more active metal will displace a less active one from its compounds 36 Predict the Products of the Following: • Zn + CuSO4→ • Cu + ZnSO4 → • AgNO3(aq) + Cu(s) → • Solid magnesium and aqueous iron(III) chloride • Solid nickel and aqueous sodium chloride 37 Predict the Products of the Following: • Zn + CuSO4→ Cu(s) + ZnSO4(aq) • Cu + ZnSO4 → NR • AgNO3(aq) + Cu(s) → Ag(s) + Cu(NO3)2 (aq) • Solid magnesium and aqueous iron(III) chloride Mg(s) + FeCl3(aq)→ MgCl2(aq) + Fe(s) • Solid nickel and aqueous sodium chloride Nis) + NaCl(aq)→ NR 5.4 A more active metal will displace a less active one from its compounds 38 Oxygen reacts with many substances The products depend, in part, on how much oxygen is available Combustion of hydrocarbons O 2 plentiful : CH 4 2O 2 CO2 2H 2O O 2 limited : 2CH 4 3O 2 2CO 4H 2 O O 2 very limited : CH 4 O 2 C 2H 2O Organic compounds containing O also produce carbon dioxide and water C2 H5OH 3O2 2CO2 3H 2O Problem Solving: A 0.3000 g sample of tin ore was dissolved in acid solution converting all the tin to tin(II). In a titration, 8.08 mL of 0.0500 M KMnO4 was required to oxidize the tin(II) to tin(IV). What was the percentage tin in the original sample? The reaction also produced MnO2 3Sn 2 2MnO -4 8H 3Sn 4 2MnO 2 4H 2O Problem Solving When 250.00 mL of 0.200M of sodium sulfite is reacted with 3.50 g of potassium dichromate (K2Cr2O7 FW=294.18) in an acidic solution, the products of the reaction include sulfate ion and chromium(III) ion. What is the concentration of chromium(III) ion if the total volume is now 251.25 mL? How would the problem be different with 5.50 g of potassium dichromate? Problem Solving A solution of sodium thiosulfate (Na2S2O3) reacts with chlorine gas in an acidic solution to give sulfate ion and chloride ion. How many mL of 0.100 M sodium thiosulfate would be needed to react with 4.25 g of chlorine gas? Problem Solving All the iron in a 2.000 g sample of iron ore is dissolved in an acidic solution and converted to iron(II) ion. When titrated with 0.100 M potassium permanganate the iron was oxidized to iron(III) and the permanganate to manganese dioxide. The titration required 27.45 ml of permanganate solution to reach the endpoint. What was the percent by mass iron in the ore? Balance in acidic solution I2 + OCl- IO3- + ClH3AsO3 + Cr2O72- H3AsO4 + Cr3+ Balance in acidic solution • VO2+ + Sn2+ VO2+ + Sn4+ Balance in basic solution • NiO2 + Mn(OH)2 Mn2O3 + Ni(OH)2 • O2 + N2H2 H2O2 + N2 In an acidic solution, Manganese(II) is oxidized to MnO4- by bismuthate ion (BiO3- ). In the reaction, BiO3- is reduced to Bi3+ Write a balanced net ionic equation for the reaction How many grams of NaBiO3 are needed to oxidize 25.0 mL of 0.200 M MnSO4 • Aluminum metal and oxygen gas forms aluminum oxide solid. • Solid sulfur (S8) burns in oxygen gas to make gaseous sulfur trioxide • Copper metal is heated in oxygen to form black copper(II) oxide solid. 5.5 Molecular oxygen is a powerful oxidizing agent 51 What is the coefficient on O2 when octane, C8H18 is combusted with scant oxygen? A. 1 B. 2 C. 3 D. 4 E. none of these 5.5 Molecular oxygen is a powerful oxidizing agent 52 What is the coefficient on O2 when iron combusts with plentiful oxygen available ? A. 1 B. 2 C. 3 D. 4 E. none of these 5.5 Molecular oxygen is a powerful oxidizing agent 53 Ore Analysis A 0.3000 g sample of tin ore was dissolved in acid solution converting all the tin to tin(II). In a titration, 8.08 mL of 0.0500 M KMnO4 was required to oxidize the tin(II) to tin(IV). What was the percentage tin in the original sample? 2 4 3Sn 2MnO 8H 3Sn 2MnO 2 4H 2O 4 5.6 Redox reactions follow the same stoichiometric principles as other reactions 54 A 25.0 g sample of granite contains a vein of copper. What is the % of Cu present if 25.00 mL of concentrated 15 M HNO3 are reacted? Cu(s) + 2NO3-(aq) + 4H+(aq) →2NO2(g) + 2H2O(l) + Cu2+ A. 12 B. 38 C. 48 D. 95 E. none of these 5.6 Redox reactions follow the same stoichiometric principles as other reactions 55