* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Thermophotovoltaic wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Degenerate matter wikipedia , lookup
Temperature wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Transition state theory wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Eigenstate thermalization hypothesis wikipedia , lookup
Heat transfer physics wikipedia , lookup
History of thermodynamics wikipedia , lookup
Thermodynamics wikipedia , lookup
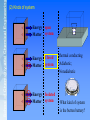
Chapter 1 The first law of thermodynamics §1.1 Basic introduction 1.1 Thermodynamics: What is chemical thermodynamics? A branch of physical chemistry that studies the energy conversion during chemical processes. A macroscopic science, the study of two physical quantities, energy and entropy. Particularly concerns with the interconversion of energy as heat and work. Problem: find the three definitions of thermodynamics in the textbook. Energy: reservation and conversion What is energy? Energy is the capacity to do work or to produce heat. Electricity: CO2, SO2 coal (chemical energy) combustion (in a burner, heat / thermal energy is produced) expansion of gas (drives piston in a turbine, work, mechanical energy) electricity (rotator in generator, electric energy) Transportation: CO2, NOx oil (chemical energy) combustion (burn in an engine, heat, thermal energy) expansion of gas (work, mechanical energy) movement (dynamic energy) How do we study the transfer of energy? The problem was put forward due to study of thermal machine: turbine. Heat out Work in / out Heat in High T To power our modern civilization, we Heat flow Work need to know the relationship between chemistry and energy. Low T 1.2 Some basic concepts (1) System and surroundings System: The parts of universe under study. Surroundings: The parts of the universe that interacts with the system Boundary/wall: real or imaginary; rigid or nonrigid, permeable or impermeable Water: open system Cup: open system Box: closed /isolated system Selection of system (2) Kinds of system Energy open Matter system Energy Closed Matter system Energy Matter Isolated system thermal conducting Adiabatic; Nonadiabatic What kind of system is the button battery? (3) System at equilibrium: the way we define the system 1) Mechanical equilibrium Four Equilibriums 2) Thermal equilibrium 3) Chemical equilibrium 4) Phase equilibrium Equilibrium thermodynamics System at equilibrium: p, T, c The properties of the system such as the pressure (p), temperature (T), composition and concentration (c, and pB) and the number of phases do not change with time. (3) State and state functions The overall behavior of the system is state. The physical and chemical quantities used to describe the state of the system is state function. example 1 mol of hydrogen gas at 1 p and 273.5 K, with the volume of 22.4 dm3 and mass of 2 g. State functions used for describe the system: Composition: mass (m), number of substance (n), Geometric: area (A), volume (V) ; Mechanical: pressure (p), surface tension () , density() Chemical: the amount of substance (n), molality (m), molarity (c), molar fraction (x) Electromagnetic: current density (I), strength of electric field (E) ; Thermodynamic: temperature (T), enthalpy (H), internal energy (U), Holmholtz’s function (F), Gibbs’ function (G) The zeroth law of thermodynamics: Definition of temperature Extensive property : The value of the property changes according to the amount of substance which is present (e.g., mass, volume, internal energy) Intensive property : The value of the property is independent of the amount of substance which is present (e.g., temperature, density) Properties Quantity extensive intensive Volume (V), the Pressure (p), concentration (c), amount of substance density (), heat capacity (C), (n), mass (m), dielectric constant (), etc. Ratio Molar mass (M), molar volume (V) Scalar or vector We usually don’t consider electric, magnetic, gravitational field Is there any relationship between state functions? 1 mol of hydrogen gas at 1 p and 273.5 K, with the volume of 22.4 dm3 and mass of 2 g. pV nRT V V ( p, T ) Need we define all the state functions of a system to describe the system? Basically, we can define the state of a single-component system using only three state functions: the amount of substance, pressure and temperature, i.e., n, T, p. For a closed single-component system with known amount of substance, we need only pressure and temperature, i.e., T, p. For a multi-component system, we need the amount of each component, n1, n2nS, and pressure and temperature. One extensive property and two intensive properties. Important properties of state function 1) The value of a particular state function for a system depends solely on the state of the system. Once the state is set, all the state functions will have a definite value. And the state function difference between two different states only depends on the initial and final state of a process. 4m For state function A glass of water is now at 50 oC. Did it cool from 100 oC? Or was it heated from 25 oC? No one knows! Future ? History ? p1, T1 V V ( p, T ) dV dV dV dp dT dT dT p dp T State functions have overall differential. (4) Path functions: A property depends upon the path by which a system in one state is changed into another state . 4m Are you strong enough to jump 4 m high in one jump? Certainly not. But I can attain that height step by step! (5) Processes: p 2 , T1 isotherm Isobar Isotherm; Isobar; Cycle; Reversible; Adiabatic Initial state p 1 , T1 Final state p 2 , T2 isotherm Isobar p 1 , T2 Summary System vs. surroundings Classification of systems: open, closed, isolated; System equilibriums: mechanical, temperature, chemical and phase State and state function: Extensive state function vs. intensive state function state function vs. process function Processes: isotherm, isobar, cycle, reversible, adiabatic