* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture notes - Science with Ms. Reathaford!
Survey
Document related concepts
Transcript
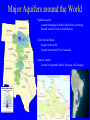
GROUNDWATER Chapter 10 Section 10.1 Movement and Storage of Groundwater • Although groundwater is present everywhere beneath the surface of the land, it is a small percentage of the water found on Earth • Groundwater is water under Earth’s surface in soil or rock • Porous materials can hold more water. The percentage of pore space in a material is referred to as porosity. The greater the porosity, the more water can infiltrate into the ground. The Water Table • Zone of Aeration: Area between water table and surface that can still hold more water. AKA: unsaturated zone • Zone of Saturation: Area where all the pore spaces in the ground are filled with water • Recharge: Water from precipitation and runoff is added back to the zone of saturation through this process • Water Table: Surface of the zone of saturation Cone of Depression Sinking of the water table around a well due to pulling out water faster than the ground can handle Groundwater Movement and Flow • Porosity is the percentage of pore space in a material. The greater the porosity, the more water can infiltrate the ground. • Aquifers are permeable layers through which most ground water flows (typically sand, gravel, sandstone, limestone) • • • Aquicludes are impermeable layers that act as barriers to ground water flow (typically clay or shale) The ability of a material to let water pass through is referred to as permeability. How quickly groundwater flows is dependent upon the permeability of the material and slope of the water table. Major Aquifers around the World Ogallala Aquifer Located throughout Central United States, stretching beneath western Texas to South Dakota Great Artesian Basin Largest in the world Located underneath 22% of Australia Guarani Aquifer Located in Argentina, Brazil, Paraguay, and Uruguay Section 10.2 Groundwater Erosion and Deposition Most groundwater contains some acid, in most cases carbonic acid. Carbonic acid forms when carbon dioxide dissolves in water and combines with water molecules. As result, groundwater is usually acidic (slightly) and attacks carbonate rocks (limestone), which readily dissolves in any kind of acid. Caves / Caverns Caves form when the acids in groundwater dissolve calcitic rocks & minerals Stalactites Calcite deposits that hang down from the ceiling of a cave as water evaporates Stalagmites Calcite deposits that grow UP from the floor of a cave as water evaporates Traverse Columns: When the stalagmites and stalactites drop or build to heights were they connect to one another. Sometimes, water is discharged at Earth’s surface where an aquifer and aquiclude come in contact. This is referred to as a spring. Spring water is usually though of as being cool and refreshing; however, the temperature of the groundwater that is discharged is typically the average annual temperature of the region in which it is located. Sometimes, springs discharge water that is much warmer than the average annual temperature because they are deep within the Earth’s crust or near igneous activity. These are called hot springs. The Liard Hot Springs along the Alaska Highway Geysers Geysers are boiling hot springs that periodically erupt as gushers of hot water and steam. Geysers erupt at regular intervals. One of the world’s most famous geyser, Old Faithful, erupts approximately every hour with a 40m high column of boiling water / steam. Wells Normal wells are holes dug deep enough to reach the water table, then water is pumped up. Types of Wells Artesian Wells When water naturally rises to the surface Pumped Wells Requires pressure and/or energy to get water to the surface Threats to our Water Supply 1. Chemicals and Pollutants Water Table aquifers are easily polluted by surface spills of pollutants, sewage leaks, industrial waste, landfills, etc. By building confined aquifers, we are protecting them from pollutants as they lie between two aquicludes 2. Overuse Groundwater supplies can be depleted if pumped out at a rate greater than the recharge rate, the groundwater supply will decrease and the water table will drop. 3. Subsidence Excessive withdrawal of water causes the sinking of land Conserving Groundwater • Overuse of groundwater causes the water table to sink, which makes wells run dry. • Pollutants that seep into groundwater are very difficult to remove. • The solution? Pump water back into our aquifers as we use it, and prevent harmful pollutants from getting there in the first place. Water Types Hard Water Contains a substantial amount of ions (calcium or magnesium) that were dissolved from minerals. Both are classified as contaminants, but we actually need them in our diet! The dissolved minerals interfere greatly with hard water’s use in nearly all aspects of the home: • Laundered clothes may look dingy and feel scratchy • Dishes and glasses may appear spotted when dry • Hair may feel sticky / straw-like • Water pressure may be low due to pipe residue While hardwater can be a nuisance in the home there are no reports indicating it is bad for you to drink. In actuality, extremely hard water may be beneficial in adding daily amounts of calcium and magnesium to your diet Soft Water Contains few or no contaminants and is either naturally occurring or can be produced through use of water treatment devices Does not interfere with household soaps and cleansers, so is not as great of a nuisance in your home: • Reduces need for detergent by nearly 50% • Soap lathers easily, providing a more effective experience for your skin • Prevents deposits left on pots, pans, and dishes when cleaning • Cuts overall energy costs due to its being more effective in cleaning Soft water can be a nuisance in some areas of home ownership, despite the clear advantages listed above: • Can deteriorate metal equipment used in swimming pools • Can stain concrete, vinyl and fiberglass materials • Reduces the effectiveness of chlorine Concentration of hardness as a calcium carbonate across the United States