* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Gastroenteritis wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
Common cold wikipedia , lookup
Hepatitis B wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Urinary tract infection wikipedia , lookup
Carbapenem-resistant enterobacteriaceae wikipedia , lookup
Neonatal infection wikipedia , lookup
Clostridium difficile infection wikipedia , lookup
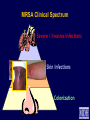
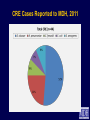
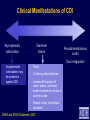
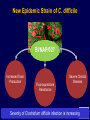
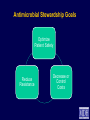
Infection Prevention & Control of Multidrug-resistant Organisms in Ambulatory Surgical Centers Jane Harper, RN, MS, CIC Lindsey Lesher, MPH Minnesota Department of Health Acute Disease Investigation and Control Section Objectives • Describe multidrug-resistant organisms (MDRO) • Describe MDRO surveillance trends in Minnesota • Describe MDRO infection prevention and control measures in ambulatory surgical centers • Describe antibiotic resistance Drug-resistant 'superbugs' hit 35 states, spread worldwide By Steve Sternberg, USA TODAY Sept. 17, 2010 Bacteria that are able to survive every modern antibiotic are cropping up in many U.S. hospitals and are spreading outside the USA, public health officials say. The bugs, reported by hospitals in more than 35 states, typically strike the critically ill and are fatal in 30% to 60% of cases. Israeli doctors are battling an outbreak in Tel Aviv that has been traced to a patient from northern New Jersey, says Neil Fishman, director of infection control and epidemiology at the University of Pennsylvania and president of the Society of Healthcare Epidemiologists. The bacteria are equipped with a gene that enables them to produce an enzyme that disables antibiotics. The enzyme is called Klebsiella pneumoniae carbapenamase, or KPC. It disables carbapenam antibiotics, last-ditch treatments for infections that don't respond to other drugs. Multi-drug Resistant Organisms (MDRO) • Bacteria that acquire the ability to resist treatment against more than one antibiotic • Infections caused by MDRO: – More difficult to treat; require more toxic antibiotics – Often result in poor patient outcomes – Cost more • MDRO are readily transmitted in healthcare settings SSI: surgical site infection CLABSI: central line-associated bloodstream infection VAP: ventilator-associated pneumonia CAUTI: catheter-associated urinary tract infection Source: CDC Staphylococcus aureus • ~ 20% of humans are persistently colonized (children > adults); ~ 60% are intermittently colonized • Most often spread via contaminated hands Methicillin-Resistant S. aureus (MRSA) • Resistant to beta-lactam antibiotics (all penicillins and cephalosporins) • Identified based on antimicrobial susceptibility testing MRSA Clinical Spectrum Severe / Invasive Infections Skin Infections Colonization “Types” of MRSA • Community–associated (CA-MRSA) – Skin infections common – No recent hospitalization, dialysis, surgery, LTCF residence – Susceptible to most antibiotics except beta-lactams and erythromycin • Healthcare-associated (HA-MRSA) – Causes nosocomial pneumonia, surgical wound, and bloodstream infections – Risk factors: hospitalization, LTCF resident, dialysis, surgery – Resistant to many antimicrobials MRSA Cases Reported to MDH, 2000-2009 4,000 56% CAMRSA Total MRSA CA-MRSA Total No. of Cases 3,500 3,000 2,500 12% CAMRSA 56% 2,000 51% 41% 1,500 53% 34% 1,000 500 12% 12% 14% 18% 22% 0 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 Year Carbapenem-Resistant Enterobacteriaceae (CRE) Carbapenems • Class of antibiotics • Mainstay of treatment targeting resistant Gramnegative bacilli • Ertapenem, imipenem, meropenem, doripenem Enterobacteriaceae • Large family of Gram-negative bacteria • Common species – – – – Klebsiella pneumoniae Escherichia coli Enterobacter cloacae Enterobacter aerogenes Carbapenem-Resistant Enterobacteriaceae (CRE) • Resistant to ≥3 classes of antibiotics, including carbapenems • Resistance mechanisms – Enzymes that inactivate carbapenems • Klebsiella pneumoniae carbapenemase (KPC) • New Delhi Metallo β-lactamase (NDM-1) – Located on chromosomes or plasmids (mobile genetic elements) CRE Cases Reported to MDH, 2011 What kinds of infections do CREs cause? • Urinary tract, intestinal or abdominal, respiratory tract, and wound infections • Most frequently isolated from urine, sputum, or blood • Bloodstream infections are associated with higher rates of death than infection at other sites Patel JB. Presented at 107th ASM General Meeting, 2007 Agmon O. Presented at 8th Congress of IFIC. 2007 Who is at increased risk for infection with CREs? • Hospitalized patients with: – – – – Co-morbid conditions Frequent or prolonged hospitalization Invasive devices Antimicrobial exposure (vancomycin, fluoroquinolones, penicillins, and extended-spectrum cephalosporins) Esther T. Tan, et al. CID. Submitted Clostridium difficile (C. diff) C. difficile Bacteria • Named due to difficulty to isolate in the lab (Latin difficile = difficult) • Spore-forming, anaerobic, gram-positive bacillus • Fecal-oral transmission – Hands of healthcare personnel – Contaminated inanimate objects • Two major reservoirs: – Infected humans (symptomatic or colonized) – Inanimate objects CDC Fact Sheet, 2005 Simor ICHE, 2002 C. difficile Infection (CDI) Facts Hospital stays from C. difficile infections tripled in the last decade, posing a patient safety threat especially harmful to older Americans. Almost all C. difficile infections are connected to getting medical care. Hospitals following infection control recommendations lowered C. difficile infection rates by 20% in less than 2 years. CDC. http://www.cdc.gov/vitalsigns/hai/ Risk Factors for C. difficile Infection • • • • • • • • • Main modifiable Antimicrobial exposure risk factors Acquisition of C. difficile Advanced age Underlying illness Immunosuppression Tube feeds Gastric acid suppression Use of nasogastric or gastrostomy feeding tubes Use of proton-pump inhibitors CDI and Antibiotic Use – > 90% cases occur during or after antibiotic therapy – All antibiotics implicated; • Broad spectrum agents are more likely associated Clinical Manifestations of CDI Asymptomatic colonization Diarrheal illness Pseudomembranous colitis Toxic megacolon Asymptomatic colonization may be protective against CDI • Fever • Cramping abdominal pain • Increased frequency of loose, watery, unformed bowel movements not due to another cause • Recent history of antibiotic exposure SHEA and IDSA Guidelines, 2007 New Epidemic Strain of C. difficile BI/NAP/027 Increased Toxin Production Fluoroquinolone Resistance Severe Clinical Disease Severity of Clostridium difficile infection is increasing MDH CDI Surveillance • Population- & laboratory-based surveillance – Four central MN counties: Benton, Stearns, Morrison, Todd – Olmsted county MDH CDI Surveillance, 2011 Percent of total cases Median age (years) Community-onset, no healthcare exposure 63% 49 Nosocomial (onset >3 days after hospital admit) 19% 79 Community-onset with healthcare exposure 18% 57 CDI case category Summary of MDRO Surveillance • Infections caused by MDRO are increasing • MDRO infections require more toxic, expensive antibiotics and result in increased adverse drug reactions • MDRO threaten the effectiveness of existing antimicrobials • MDRO are transmissible in healthcare settings and the community Ambulatory Surgical Centers • Increasing and more complex, invasive care provided in ambulatory care facilities • Procedures performed in ASC: – 1996: 32 million – 2006: > 53 million • From 1996 to 2006: – 273% increase in spinal cord injections (increase of 1.5 million) – 200% more colonoscopies (increase of 4 million) Data from the National Survey of Ambulatory Surgery GAO. HHS Has Taken Steps to Address Unsafe Injection Practices, but More Action Is Needed. 2012. Increased Awareness of Infection Prevention & Control Needs in ASCs Government Accountability Office (GAO) Report (2009) “The increasing volume of procedures and evidence of infection control lapses in ASCs create a compelling need for current and nationally representative data on HAIs in ASCs in order to reduce their risk…” Infection Prevention & Control Strategies • • • • • Standard Precautions Isolation Precautions Hand hygiene Injection safety Cleaning and high-level disinfection/sterilization of reusable medical equipment Standard Precautions • Basic level of infection control precautions for use in the care of all patients • Applies to: – Blood and all body fluids, secretions and excretions – Non-intact skin – Mucous membranes • Personal protective equipment as indicated by patient/procedure/situation • Hand hygiene - always! • Respiratory hygiene / cough etiquette Transmission-based Precautions Standard Precautions + – Contact • Direct (skin to skin, fecal-oral) and indirect (environmental) • Gloves, gown (if splashing, contamination is possible) • E.g. MRSA, CRE – Droplet • Large droplets: respiratory secretions, coughing, sneezing • Surgical mask within 3-6 feet of patient • E.g. Pertussis, influenza – Airborne • Pathogens suspended in air as small particles • N95, PAPR, negative pressure room • E.g. Tuberculosis, varicella Hand hygiene • Perform hand hygiene: – After touching blood, body fluids, secretions, excretions, etc. – – • whether or not gloves were worn Immediately after removing gloves Between patient contacts • Antimicrobial soap and water / friction • Alcohol-based hand rubs Caveat: Organic material inactivates alcohol, must wash to remove visible soil Standard Precautions: Injection Safety • Practices that prevent contamination during preparation and administration of all parenteral medications CDC 2007 Guideline for Isolation Precautions www.cdc.gov/hicpac/pdf/isolation/Isolation2007.pdf 18 Outbreaks of Viral Hepatitis Associated with Unsafe Injection Practices in Ambulatory Settings, 2001-2011 Healthcare Setting # of Outbreaks Pain management clinics 5 Endoscopy clinics 5 Alternative medicine clinics 3 Hematology-oncology clinics 2 • 2 common unsafe injection practices that resulted in BBP transmission (both can transmit infections, even if the needle is changed): – Reuse of a syringe for multiple patients – Accessing a medication vial used for multiple patients Source: CDC CDC One and Only Campaign • Promote safe injection practices • Provide information to clinicians in all types of healthcare settings Unsafe injection practices = never events Injection safety training video www.oneandonlycampaign.org/videos/Default.aspx CDC injection safety FAQs from providers www.cdc.gov/injectionsafety/ Cleaning and Disinfection/Sterilization: Reusable Medical Equipment • Reusable medical equipment must be appropriately cleaned and disinfected / sterilized prior to each use – Glucometers, other point of use devices – Endoscopes – Surgical instruments • Assign responsibilities and ensure annual competency training and education • Use appropriate PPE when handling/reprocessing contaminated equipment Endoscope Reprocessing Breaches Reported to MDH, 2010-2011 No. Healthcare facility type Ambulatory surgical center Clinic Hospital 1 1 5 Breaches that resulted in patient notification 4 Number of patients affected Cause of breach Lack of communication between reprocessing departments within facility Endoscope owned by physician; facility did not take responsibility for regular maintenance and staff training Reprocessing of single use device following incorrect instructions provided by vendor representative Failure to follow manufacturer instructions resulted in use of incorrect AER connector 6 - 2,600 Piece of cleaning brush dislodged into patient's colon procedure Use of improper AER connector due to incorrect manufacturer instructions Unknown 1 1 1 1 1 1 1 MDH Poster Key Endoscope Reprocessing Steps www.health.state.mn.us/divs/idepc/dtopics/infectioncontrol/scope/ Antimicrobial Stewardship Goals Optimize Patient Safety Reduce Resistance Decrease or Control Costs Antimicrobial Stewardship Strategies Right drug/dose/duration Obtain cultures/avoid empiric prescribing if possible Adjust empiric prescribing/stop antibiotic based on lab results Antimicrobial Stewardship Programs • Provide the infrastructure to preserve antimicrobials • Promote patient safety • Can be implemented in any healthcare setting – from the smallest to the largest • CDC: Get Smart About Antibiotics in Healthcare http://www.cdc.gov/getsmart/healthcare/?s_cid=dhqp_002 Minnesota Guide to a Comprehensive Antimicrobial Stewardship Program New! www.health.state.mn.us/divs/idepc/dtopics/antibioticresistance/index.html Resources Available From the MDH Website http://www.health.state.mn.us/ Two components: • SAFE = Infrastructure to support SSI Prevention Strategies • SSI Prevention Strategies = “CUTS” www.health.state.mn.us/divs/idepc/dtopics/hai/ssi/toolkit/index.html SAFE CUTS • SSI Prevention Team – Champion, inter-disciplinary team • Access to Information – Audit prevention steps (e.g., a pre-, intra-, post-procedure checklist – Measure outcomes (National Healthcare Safety Network [NHSN]) • Facility Expectations – Process for speaking up and “stopping the line” – Communicate expectations to all providers: pre-op evaluation for infections; postpone elective surgery until infection resolved • Education – Clinicians and staff – Patients (pre-op, post-op) SAFE CUTS (cont.) • Cleaning surgical equipment/environment – Appropriate use of immediate use sterilization • Undergoing surgery – Pre-op: antibiotics, pre-warming, blood glucose, skin prep – During: Keep OR door closed, maintain normothermia – Post-op: Normothermia, blood glucose, patient /family education • Team Accountability/Communication – pre-op briefing, surgical checklist to track SSI prevention measures • Staff – Expectations: hand hygiene, illness, surgical attire SAFE From CDI www.health.state.mn.us/divs/idepc/diseases/cdiff/toolkit/index.html MRSA Patient Education www.health.state.mn.us/divs/idepc/diseases/mrsa/index.html CDC Resources • CDC Management of Multidrug-Resistant Organisms In Healthcare Settings, 2006 www.cdc.gov/hicpac/mdro/mdro_0.html • CDC, Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care and the Infection Prevention Checklist for Outpatient Settings: Minimum Expectations for Safe Care www.cdc.gov/HAI/settings/outpatient/outpatient-care-guidelines.html • See CDC, Basic Infection Control and Prevention Plan for Outpatient Oncology Settings, accessed March 1, 2012, www.cdc.gov/HAI/settings/outpatient/basic-infection-control-prevention-plan2011/index.html • Get Smart: Know When Antibiotics Work www.cdc.gov/getsmart/ Summary • MDRO are a growing challenge in all areas of healthcare • Early identification of MDRO and implementation of infection prevention and control measures is effective in limiting transmission • Antimicrobial stewardship is a critical component of MDRO prevention • Patient education is an important MDRO prevention measure in ambulatory care • Compliance with infection prevention measures is essential Questions? Minnesota Department of Health website www.health.state.mn.us/divs/idepc/diseases/mrsa/index.html MDH Acute Disease Investigation and Control 651-201-5414 or toll-free 1-877-676-5414 Thank You!