* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture 2: Applications of Tissue Culture to Plant Improvement
Survey
Document related concepts
Transcript
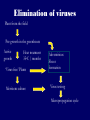
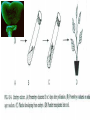
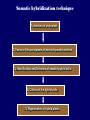
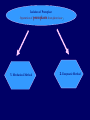
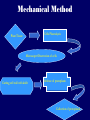
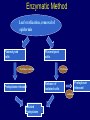
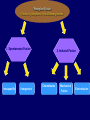
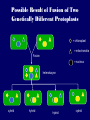
Plant Tissue Culture Application Development of superior cultivars Germplasm storage Embryo rescue Ovule and ovary cultures Anther and pollen cultures Callus and protoplast culture Protoplasmic fusion Plant Genetic Engineering Tissue Culture Applications Micropropagation Germplasm preservation Somaclonal variation Haploid & dihaploid production In vitro hybridization – protoplast fusion Plant genetic engineering Features of Micropropagation • Clonal reproduction – Way of maintaining heterozygozity • Multiplication stage can be recycled many times to produce an unlimited number of clones – Routinely used commercially for many ornamental species, some vegetatively propagated crops • Easy to manipulate production cycles – Not limited by field seasons/environmental influences • Disease-free plants can be produced – Has been used to eliminate viruses from donor plants COMPARISON OF CONVENTIONAL & MICROPROPAGATION OF VIRUS INDEXED REGISTERED RED RASPBERRIES Conventional Micropropagation Duration: 6 years 2 years Labor: Dig & replant every 2 years; unskilled (Inexpensive) Subculture every 4 weeks; skilled (more expensive) Space: More, but less expensive (field) Less, but more expensive (laboratory) Required to prevent viral infection: Screening, fumigation, spraying None Ways to eliminate viruses Heat treatment. Plants grow faster than viruses at high temperatures. Meristemming. Viruses are transported from cell to cell through plasmodesmata and through the vascular tissue. Apical meristem often free of viruses. Trade off between infection and survival. Not all cells in the plant are infected. Adventitious shoots formed from single cells can give virusfree shoots. Elimination of viruses Plant from the field Pre-growth in the greenhouse Active growth Heat treatment 35oC / months ‘Virus-free’ Plants Meristem culture Adventitious Shoot formation Virus testing Micropropagation cycle Storage of Plant germplasm In situ : Conservation in ‘normal’ habitat –rain forests, gardens, farms Ex Situ : –Field collection, Botanical gardens –Seed collections –In vitro collection: Extension of micropropagation techniques •Normal growth (short term storage) •Slow growth (medium term storage) •Cryopreservation (long term storage DNA Banks In vitro Collection Use : Recalcitrant seeds Vegetatively propagated Large seeds Concern: Security Availability cost Ways to achieve slow growth Use of immature zygotic embryos (not for vegetatively propagated species) Addition of inhibitors or retardants Manipulating storage temperature and light Mineral oil overlay Reduced oxygen tension Defoliation of shoots Cryopreservation Storage of living tissues at ultra-low temperatures (-196°C) Conservation of plant germplasm • Vegetatively propagated species (root and tubers, ornamental, fruit trees) • Recalcitrant seed species (Howea, coconut, coffee) Conservation of tissue with specific characteristics • Medicinal and alcohol producing cell lines • Genetically transformed tissues • Transformation/Mutagenesis competent tissues (ECSs) Eradication of viruses (Banana, Plum) Conservation of plant pathogens (fungi, nematodes) Cryopreservation Steps Selection Excision of plant tissues or organs Culture of source material Select healthy cultures Apply cryoprotectants Pregrowth treatments Cooling/freezing Storage Warming & thawing Recovery growth Viability testing Post-thawing Cryopreservation Requirements • Preculturing – Usually a rapid growth rate to create cells with small vacuoles and low water content • Cryoprotection – Cryoprotectant (Glycerol, DMSO, PEG) to protect against ice damage and alter the form of ice crystals • Freezing – The most critical phase; one of two methods: • Slow freezing allows for cytoplasmic dehydration • Quick freezing results in fast intercellular freezing with little dehydration Cryopreservation Requirements • Storage – Usually in liquid nitrogen (-196oC) to avoid changes in ice crystals that occur above -100oC • Thawing – Usually rapid thawing to avoid damage from ice crystal growth • Recovery – Thawed cells must be washed of cryo-protectants and nursed back to normal growth – Avoid callus production to maintain genetic stability Somaclonal Breeding Procedures • Use plant cultures as starting material – Idea is to target single cells in multi-cellular culture – Usually suspension culture, but callus culture can work (want as much contact with selective agent as possible) – Optional: apply physical or chemical mutagen • Apply selection pressure to culture – Target: very high kill rate, you want very few cells to survive, so long as selection is effective • Regenerate whole plants from surviving cells Requirements for Somaclonal Breeding • Effective screening procedure – Most mutations are deleterious • With fruit fly, the ratio is ~800:1 deleterious to beneficial – Most mutations are recessive • Must screen M2 or later generations • Consider using heterozygous plants? – But some say you should use homozygous plants to be sure effect is mutation and not natural variation • Haploid plants seem a reasonable alternative if possible – Very large populations are required to identify desired mutation: • Can you afford to identify marginal traits with replicates & statistics? Estimate: ~10,000 plants for single gene mutant • Clear Objective – Can’t expect to just plant things out and see what happens; relates to having an effective screen – This may be why so many early experiments failed Embryo Culture Uses • Rescue F1 hybrid from a wide cross • Overcome seed dormancy, usually with addition of hormone to media (GA) • To overcome immaturity in seed – To speed generations in a breeding program – To rescue a cross or self (valuable genotype) from dead or dying plant Haploid Plant Production Embryo rescue of interspecific crosses – Creation of alloploids Anther culture/Microspore culture – Culturing of Anthers or Pollen grains (microspores) – Derive a mature plant from a single microspore Ovule culture – Culturing of unfertilized ovules (macrospores) Specific Examples of DH uses • Evaluate fixed progeny from an F1 – Can evaluate for recessive & quantitative traits – Requires very large dihaploid population, since no prior selection – May be effective if you can screen some qualitative traits early • For creating permanent F2 family for molecular marker development • For fixing inbred lines (novel use?) – Create a few dihaploid plants from a new inbred prior to going to Foundation Seed (allows you to uncover unseen off-types) • For eliminating inbreeding depression (theoretical) – If you can select against deleterious genes in culture, and screen very large populations, you may be able to eliminate or reduce inbreeding depression – e.g.: inbreeding depression has been reduced to manageable level in maize through about 50+ years of breeding; this may reduce that time to a few years for a crop like onion or alfalfa Somatic Hybridization Development of hybrid plants through the fusion of somatic protoplasts of two different plant species/varieties Somatic hybridization technique 1. isolation of protoplast 2. Fusion of the protoplasts of desired species/varieties 3. Identification and Selection of somatic hybrid cells 4. Culture of the hybrid cells 5. Regeneration of hybrid plants Isolation of Protoplast (Separartion of 1. Mechanical Method protoplasts from plant tissue) 2. Enzymatic Method Mechanical Method Cells Plasmolysis Plant Tissue Microscope Observation of cells Cutting cell wall with knife Release of protoplasm Collection of protoplasm Mechanical Method Used for vacuolated cells like onion bulb scale, radish and beet root tissues Low yield of protoplast Laborious and tedious process Low protoplast viability Enzymatic Method Leaf sterlization, removal of epidermis Plasmolysed cells Plasmolysed cells Pectinase +cellulase Protoplasm released Pectinase Protoplasm released Release of isolated cells cellulase Isolated Protoplasm Enzymatic Method Used for variety of tissues and organs including leaves, petioles, fruits, roots, coleoptiles, hypocotyls, stem, shoot apices, embryo microspores Mesophyll tissue - most suitable source High yield of protoplast Easy to perform More protoplast viability Protoplast Fusion (Fusion of protoplasts of two different genomes) 1. Spontaneous Fusion Intraspecific Intergeneric 2. Induced Fusion Chemofusion Mechanical Fusion Electrofusion Uses for Protoplast Fusion Combine two complete genomes – Another way to create allopolyploids In vitro fertilization Partial genome transfer – Exchange single or few traits between species – May or may not require ionizing radiation Genetic engineering – Micro-injection, electroporation, Agrobacterium Transfer of organelles – Unique to protoplast fusion – The transfer of mitochondria and/or chloroplasts between species Spontaneous Fusion • Protoplast fuse spontaneously during isolation process mainly due to physical contact • Intraspecific produce homokaryones • Intergeneric have no importance Induced Fusion Chemofusion- fusion induced by chemicals • Types of fusogens • • • • PEG NaNo3 Ca 2+ ions Polyvinyl alcohol Induced Fusion • Mechanical Fusion- Physical fusion of protoplasts under microscope by using micromanipulator and perfusion micropipette • Electrofusion- Fusion induced by electrical stimulation • Fusion of protoplasts is induced by the application of high strength electric field (100kv m-1) for few microsecond Possible Result of Fusion of Two Genetically Different Protoplasts = chloroplast = mitochondria Fusion = nucleus heterokaryon cybrid hybrid hybrid cybrid Identifying Desired Fusions • Complementation selection – Can be done if each parent has a different selectable marker (e.g. antibiotic or herbicide resistance), then the fusion product should have both markers • Fluorescence-activated cell sorters – First label cells with different fluorescent markers; fusion product should have both markers • Mechanical isolation – Tedious, but often works when you start with different cell types • Mass culture – Basically, no selection; just regenerate everything and then screen for desired traits Advantages of somatic hybridization • Production of novel interspecific and intergenic hybrid – Pomato (Hybrid of potato and tomato) • Production of fertile diploids and polypoids from sexually sterile haploids, triploids and aneuploids • Transfer gene for disease resistance, abiotic stress resistance, herbicide resistance and many other quality characters • Production of heterozygous lines in the single species which cannot be propagated by vegetative means • Studies on the fate of plasma genes • Production of unique hybrids of nucleus and cytoplasm Problem and Limitation of Somatic Hybridization 1. Application of protoplast technology requires efficient plant regeneration system. 2. The lack of an efficient selection method for fused product is sometimes a major problem. 3. The end-product after somatic hybridization is often unbalanced. 4. Development of chimaeric calluses in place of hybrids. 5. Somatic hybridization of two diploids leads to the formation of an amphiploids which is generally unfavorable. 6. Regeneration products after somatic hybridization are often variable. 7. It is never certain that a particular characteristic will be expressed. 8. Genetic stability. 9. Sexual reproduction of somatic hybrids. 10. Inter generic recombination. TYPICAL SUSPENSION PROTOPLAST + LEAF PROTOPLAST PEG-INDUCED FUSION NEW SOMATIC HYBRID PLANT Requirements for plant genetic transformation • Trait that is encoded by a single gene • A means of driving expression of the gene in plant cells (Promoters and terminators) • Means of putting the gene into a cell (Vector) • A means of selecting for transformants • Means of getting a whole plant back from the single transformed cell (Regeneration)