* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Survey
Document related concepts
Transcript
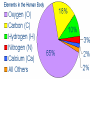
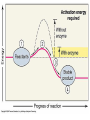
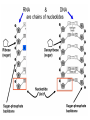
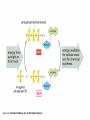
Ch. 2 Chemical Level of Organization Interactive pages 27-58 Atoms, Molecules, & Bonds Atoms are the smallest stable unit of matter Protons +charge, in the nucleus of the atom Neutrons 0charge, also in the nucleus Electrons –charge, 1/1836 the mass of a proton Atomic Structure Atomic #= # of Protons Electron cloud (orbiting eoutside the nucleus) Elements and Isotopes Elements are made of the same atom Isotopes- atoms of the same element with differing numbers of neutrons Mass #= the total number of neutrons + the total number of protons Atomic weight- avg. mass of all isotopes of that element (including electrons) Radioisotopes emit subatomic particles or radiation (radioactive decay) Half life is the time required for ½ of the given amount of isotope to decay Electrons & Energy Levels Electrons occupy a series of energy levels 1st shell (closest to the nucleus): holds 2 electrons and has the lowest energy 2nd energy level: holds up to 8 electrons 3rd: up to 18 electrons Outermost energy level is the surface of the atom and determines bonding or chemical properties of that element Chemical Bonds How elements are held together or interact Ionic Electron Donor & Electron Acceptor NaCl Covalent Share e- to form a molecule Single, double, or triple bonds Non-polar= equal sharing of electrons Polar: unequal sharing of electrons H20 Hydrogen Bonds between H+ and – N or O ends of adjacent molecules Creates surface tension Chemical Reactions When new bonds form or break between atoms Reactants Products Reactions in cells or tissue constitute metabolism Activation energy: amount of energy needed to start a reaction Enzymes (proteins): reduce activation energy; they are catalysts (speed up reaction) Types of Reactions Decomposition Hydrolysis-break down by adding water Catabolism- break down of complex molecules in the body (releases energy) Growth, movement, reproduction Synthesis Dehydration or condensation Anabolism: synthesis of new molecules within the cells of the body (requires energy) Inorganic Compounds Part II Nutrients Essential elements obtained from diet Metabolites: molecules that can be synthesized or broken down in our bodies Inorganic-no Carbon or Hydrogen in primary structure Most important in body CO2 O2 H2O Acids, bases, & Salts Water Properties Solubility (universal solvent due to polarity) Reactivity (dehydration & hydrolysis) High Heat Capacity (retains lots of heat before changing state): perspiration Lubrication (little friction between molecules Aqueous solutions Ionic bonds dissociate in water and create ions (conduct electricity) Electrolytes: inorganic molecular ions in the body vital to function & regulated by the kidneys, digestive system, & skeletal system Colloid: solution with dispersed proteins & large particles Suspension: large particles that may settle out of solution (blood) pH Dissociation of H+ ions in water making H+ and OH- (hydroxide) Neutral= 7 (pure water) equal amounts of H+ and OHAcidic= 7 more H+ than –OH Acids- proton donors releases H+ in solution (HCL) Bases- proton acceptor releases –OH in solution (NaOH) Basic= 7 less H+ than –OH (A.K.A. Alkaline) Blood has a pH of 7.35-7.45 Higher pH results in uncontrollable muscle contractionsAlkalosis Lower pH can result in coma- Acidosis Salts: Inorganic compounds made of any cation except H+ and any anion except –OH (NaCl) Buffers: stabilize pH, usually a weak acid combined with a related salt (NaHCO3- sodium bicarbonate) Organic & High Energy Compounds Part III Carbohydrates C,H,O in a 1:2:1 ratio Sugars and Starches Water soluble (hydrophilic) Monosaccharide: simple sugars Disaccharides: sucrose Polysaccharides: cellulose, starch, & glycogen (stored in muscle) Lipids Fats, oils, & waxes C,H, O (less O than in carbs) Most insoluble in water (Hydrophobic) Twice as much energy as carbs Glycerol & a fatty acid chain (saturated or unsaturated) Eicosanoids: prostigalandins released by damaged tissue to stimulate nerve endings Glycerides: energy source, insulation, protection Steroids Cholesterol: maintains cell membranes Steroids: regulation of development and tissue metabolism Phospholipids: maintain membranes Proteins Most abundant organic compound of the body (20% body weight) C, H, O, N 2 million different Functions: support, movement, transport, buffering, metabolic regulation, coordination, control, defense Structure: Amino group Carboxylic acid group R-group Nucleic Acids C, H, O, N, P Store and process information in the cell DNA & RNA Subunits or monomers called nucleotides Nucleotide made up of a pentose (5 C sugar), phosphate group, and nitrogenous base Adenine, guanine, cytosine, and thymine (or uracil in RNA) High Energy Compounds Covalent bond that harnesses energy ADP (adenosine diphosphate) Undergoes phosphorylation (attachment of a phosphate group) Requires phosphate group Enzyme to catalyze reaction Organic substrate to add phosphate to Becomes Adenosine Triphosphate (ATP) When phosphate is removed energy is released