* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Hydrogen Peroxide-Dependent Conversion of
Survey
Document related concepts
Transcript
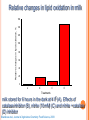
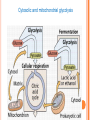
Hydrogen Peroxide-Dependent Conversion of Nitrite to Nitrate as an Essential Feature of Bovine Milk Catalase Nissim Silanikove, Agricultural Research Organization, Institute of Animal Science, Israel. Gabriel Leitner, The Veterinary Institute, Israel Scenario of NO cycling and metabolism in mammary secretion (Free radicals Biol Med, 2005) Xanthine dose-dependently enhance the conversion of nitrite to nitrate. 45 40 No xanthine Nitrite concentration, M 35 10 M xanthine 30 25 50 M xanthine 20 15 10 100 M xanthine 5 150 M xanthine 0 -5 0 10 20 30 40 50 60 Time, min Under the experimental conditions, approximately 40 µM of xanthine are converted to urate via XO within 2 h Silanikove et al, Journal of Agriculture Chemistry Food Science, 2009 50 Xa-0 Xa-10 Xa-50 Xa-100 Xa-150 M 40 30 20 10 0 Lg10 Nitrite Conc., M 0 1.8 20 40 60 80 Time, Min 1.6 1.4 1.2 1.0 rate constant min -1 x 1000 0 10 20 30 40 Time, Min 20 15 10 5 0 0 50 100 150 Xanthin concentration, M 200 Relative changes in lipid oxidation in milk Relative changes in lipid oxidation, % 160 140 120 100 80 60 40 20 0 A B C D Treatments milk stored for 6 hours in the dark at 4 0C (A), Effects of catalase inhibitor (B), nitrite (10 mM) (C) and nitrite + catalase (D) inhibitor Silanikove et al, Journal of Agriculture Chemistry Food Science, 2009 22 No addition + 30 mM 3-AT Nitrate, M 20 18 16 14 12 0 1 2 3 4 5 days 5 No addition + 30 mM 3-AT Nitrite, M 4 3 2 1 0 0 1 2 3 days 4 5 Effect of storing raw milk in the dark at 4 0C with and without catalase inhibitor Silanikove et al, Journal of Agriculture Chemistry Food Science, 2009 Nitrotyrosine, nM g-1 500 No addition + 30 mM 3-AT 400 300 200 100 0 0 1 2 4 5 days 2000 Carbonyls, nM g-1 3 No addition + 30 mM 3-AT 1500 1000 500 Effect of storing raw milk in the dark at 4 0C with and without catalase inhibitor Lipid peroxides, mEq g -1 0 0 4.0 1 2 3 4 5 days No addition + 30 mM 3-AT 3.5 3.0 2.5 2.0 0 1 2 3 days 4 5 Silanikove et al, Journal of Agriculture Chemistry Food Science, 2009 Conclusions Regarding the Control of Oxidative Stability in Milk XO and catalase works interactively as an antioxidant system Formation of nitrogen dioxide is a key process in oxidative stress in milk. Thus, controlling this process should improve milk oxidative stability The function of catalase is rate limited by hydrogen peroxide, which is provided by the activity of XO Effect of LPS: 6 cows served as control, in second set of six cows one of the front and one rear glands were treated with LPS (10 ml with 10 µg/ ml LPS) while the contra-lateral glands served as running control. The cows milk were sampled at -24h, 0 h (before treatment) and 24, 48 and 76 h posttreatment. + The data were analyzed for the effect of treatment and time at a single gland level + - Effect of LPS on milk yield 20 Milk Yield (L/day) 18 16 14 12 10 -25 0 25 50 Time in Relation to LPS Challange (h) 75 Effect of LPS on lactose concentration 5.6 A Lactose Concentration (%) 5.4 5.2 5.0 4.8 4.6 4.4 4.2 4.0 -25 0 25 50 Time in Relation to LPS Challange (h) 75 Effect of LPS on whey proteins concentration 1.0 Whey Proteins Concentration (%) B 0.9 0.8 0.7 0.6 0.5 -25 0 25 50 Time in Relation to LPS Challange (h) 75 Proteose-peptone Concentration (g/ml) Effect of LPS on proteose peptones concentration 180 C 160 140 120 100 80 60 40 -25 0 25 50 Time in Relation to LPS Challange (h) 75 Effect of LPS lactoferrin concentration 250 Lactoferrin Concentration (µg/ml) A 200 150 100 50 -25 0 25 50 Time in Relation to LPS Challange (h) 75 Effect of LPS on XO activity and urate concentration Effect of LPS on LPO activity, nitrite and nitrotyrosine concentrations Effect of LPS on catalase activity, and nitrate concentrations Updated scenario of NO-cycling in milk Novel findings: Effect of LPS on lactate, malate and citrate concentrations Cytosolic and mitochondrial glycolysis Lactic acid metabolism Cytosolic formation of malic acid 2 Pyruvic 1 acid + CO2 + ATP Pyruvate carboxylase Oxaloacetic acid + ADP 2 Oxaloacetic 1 acid + NADH + NAD 2 Malic dehydrogenase Malic acid + Low lactose and high lactic acid in broth media affect the growth of pathogenic type of E coli CONCLUSIONS The acute conversion of the epithelial cells metabolism from principally mitochondrial-oxidative to principally cytosolic (glycolysis) allows the diversion of metabolic resources normally used to synthesize milk to support the immune system. In turn, the acute increase in the concentration of lactate and malate in milk and the parallel reduction in lactose concentration are probably effective mean in restraining invading E Coli growth. SPECULTIVE CONCLUSION The main function of PMN under acute inflammation is to backup for the disruption of the epithelial tight junction integrity in order to prevent sepsis and lethality