* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download BVS stent (Abbott Vascular)
Survey
Document related concepts
Transcript
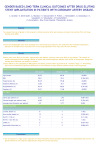
Bioabsorbable stents: early clinical results Dr Angela Hoye MB ChB, PhD Senior Lecturer in Cardiology Hull & East Yorkshire Hospitals MY CONFLICTS OF INTEREST ARE: Clinical Events Committee member for SPIRIT II, V and SPIRIT Woman, fees paid by Abbott Vascular Inc • Review the clinical evidence available Historical studies Igaki-Tamai Current players BVS AMS REVA BTI, Ideal™, Elixir Medical, Orbus etc.... Future studies Igaki-Tamai stent (Kyoto Medical Planning, Japan) • Poly-L-lactic acid (PLLA) self-expanding stent • Strut thickness of 170µm • FIM study of 50 patients (63 lesions) – 1 in-hospital stent thrombosis and Q-wave MI – 1 non-cardiac death – TLR (all with PCI): at 6 months 12% at 12 months 17% at 4 years 18% – Late loss index (in the first 15 patients) was 0.48mm at 6 months • Further studies in the SFA with this stent demonstrated feasibility and safety in deployment of these stents over a length of 70 mm – the stent has CE mark for use in PVD. Tamai et al Circulation 2000;102(4):399-404; Tamai CCT 2004 Magnesium stent (AMS, Biotronik) • Balloon-expandable magnesium alloy absorbable stent • The first clinical trial was performed in 20 patients with severe PVD with disease in the proximal two-thirds of one or more infrapopliteal arteries • All were candidates for amputation and underwent PCI with the magnesium stent on a compassionate basis • Following pre-dilatation, the 3.0×15 and 3.5×15 mm AMS were successfully deployed with good angiography and ultrasound results • At 3 and 6 months post-implantation, the stent patency rates were 89% and 78%, respectively • At 3 months, USS & MRI demonstrated complete AMS absorption • At 6 months follow-up, only 1 patient required amputation Magnesium stent (AMS, Biotronik) • • • • • Coronary FIM multicentre study of 63 patients – PROGRESS-AMS The primary endpoint was MACE at 4 months and ischemia-driven TLR Safe: no death, no MI, no stent thrombosis The stent was well-expanded on deployment with no immediate recoil High restenosis rate with an in-stent late loss of 1.08 ± 0.49mm Cumulative frequency curves of insegment luminal narrowing before, immediately after and at 4 months followup after AMS Ischaemia-driven TLR was 24% at 4 months, and 45% at 1 year Erbel et al Lancet 2007;369:1869-75 Waksman et al JACC Cardiovasc Interv. 2009 Apr;2(4):312-20 Magnesium stent (AMS, Biotronik) • PROGRESS-AMS: results of IVUS Erbel et al Lancet 2007;369:1869-75 Magnesium stent (AMS, Biotronik) “Slower degradation is warranted to provide sufficient radial force to improve long-term patency rates of the AMS” Prolong the mechanical stability in order to retain the stent throughout the period of proliferative response (AMS-2) A drug-coated version to try and suppress neointimal hyperplasia (DREAMS AMS-3) Waksman et al JACC Cardiovasc Interv. 2009 Apr;2(4):312-20 Magnesium stent (AMS, Biotronik) • • • Return of coronary vaso-reactivity following absorption 5 patients treated with AMS compared with 10 treated with metal stents QCA was measured on two orthogonal views at 0.2mm intervals throughout the stented segments and a 1cm proximal reference segment before and after administration of 2mg intra-coronary ISDN Ghimire et al Eurointervention 2008;4:481-4 REVA stent (Reva Medical) • Polycarbonate tyrosine-derived stent which breaks down to amino acids, alcohol, and carbon dioxide. It is impregnated with iodine to improve radio-opacity • Balloon-expandable and has a ratchet-like system so that as it expands the stent ratchets open and can't reclose down • Radial support for 6 months • Stent absorption over 2 years • Interim analysis of 27 FIM patients demonstrated “unfavourable results between 4 and 6 months with higher than expected TLR driven by reduced stent diameter” Grube TCT 2008 REVA stent (Reva Medical) OCT performed at 12 months demonstrated the presence of neointimal tissue covering the entire treated segment, and signs of stent absorption REVA stent (Reva Medical) • Second generation stent with sirolimus on the abluminal surface • Absorption time: 4 years • Duration strength 4-6 months • FIM due to start shortly Pollman Eurointervention Suppl 2009,F54 BVS stent (Abbott Vascular) • • • • • PLA backbone, releases everolimus (80% by 30 days) Struts are 150µm, radiopaque markers at either end ABSORB cohort A FIM trial 3x12 then 3x18mm BVS Simple lesions Ormiston et al Lancet 2008 Serruys et al Lancet 2009 BVS stent (Abbott Vascular) • • • • MACE rate was 3.4% (1 patient) No “stent” thrombosis In-”stent” late loss at 6 months was 0.43mm (0.47mm at 2 yrs) Vessel and scaffold area changes: Ormiston et al Lancet 2008 Serruys et al Lancet 2009 • Vasomotion study (n=7) • Methergine (non-endothelial dependent vasoconstrictor) • Shows the vessel is no longer “splinted” • Comparison of a Cypher stent and BVS on MSCT • Allows non-invasive FU with quantification • Results of ABSORB Cohort A were encouraging with only 1 MACE and resorption largely complete by 2 yrs • The Cohort A scaffold had shrinkage at 6 months that was the major contributor to the late loss BVS 1.1 BVS 1.0 device 1.25 0.87 – Same strut material, thickness, drug (everolimus) and resorption time – More uniform strut distribution, vessel support and drug application BVS stent (Abbott Vascular) • ABSORB Cohort B FIM trial • 3.0x18mm BVS to treat lesions ≤14mm in length BVS stent (Abbott Vascular): Cohort B FIM trial 30 days N=101 6 months N=101 Cardiac death (%) 0 0 MI (all non-Q-wave) (%) 2 3 Ischaemia-driven TLR (%) 0 2 PCI 0 2 CABG 0 0 MACE (%) 2 5 TLF (%) 2 5 No thrombosis by ARC or Protocol MACE: cardiac death, MI, ischaemia-driven TLR TLF: cardiac death, MI, ischaemia-driven TLR, ischemia-driven TVR In-”stent” late loss of BMS / Xience V / BVS 1.0 / BVS 1.1 ABSORB: BVS 1.0 Δ Vessel Area = +0.3% Δ Stent Area = -11.8% Δ Lumen Area = -16.8% NIH Area % VO (mm2) = 0.29 = 5.3% ABSORB: BVS 1.1 Δ Vessel Area = +2.4% Δ Stent Area = -2.0% Δ Lumen Area = -3.1% NIH Area (mm2) = 0.08 % VO = 1.2% • Appears safe and effective • Limitations: Markers difficult to see in large patients – Not tested in more complex anatomy – Should not re-introduce an undeployed stent as less securely mounted – Aggressive post-dilatation will cause strut fracture Summary & conclusions • Potential advantages of biodegradable stents: – Shorter duration of dual anti-platelet therapy – Recovery of endothelial function – No remaining prosthesis therefore enabling future revascularization without the increased MACE associated with treating an “in-stent restenosis”; no “full metal jacket” – Potential for applications such as angiogenesis and gene transfer – Compatibility with non-invasive imaging modalities (CTA and MRI) • The potential (long-term) advantages of biodegradable stents have to be weighed up against the limitations: – Lower strength compared to metallic stents → recoil – Relatively poor crossing profile – Radiolucent Summary & conclusions • However, we have learnt much from the early clinical studies – The importance of recoil and speed of stent absorption – The advantage of combining with anti-proliferative drug-elution • Future work will focus on optimising the trade-off between stent deliverability, strut thickness, and optimal drug-elution – – – – – BVS testing in more complex disease 2nd generation REVA which is sirolimus-eluting 2nd generation AMS with drug-elution (DREAMS) Elixir medical device (PLA sirolimus-eluting stent) Biocorrodible iron stents etc etc etc.......................