* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ACID AND BASES
Survey
Document related concepts
Transcript
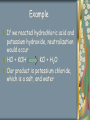
ACID AND BASES ACIDS An acid is traditionally considered any chemical compound that, when dissolved in water, gives a solution with a pH less than 7 Properties of acids: generally taste sour, are corrosive to metals, turns blue litmus paper red and reacts with a base to produce a salt and water Acids pH level ranges from 0 to 6 An acid with a pH of 0 is a very strong acid An acid with a pH of 6 is a weaker acid Examples of acids: lemon juice, vinegar, HCl BASES A base is traditionally considered any chemical compound that, when dissolved in water, gives a solution with a pH greater than 7 Properties of bases: have a bitter taste, feel slimy or soapy, are not corrosive to metals, turns red litmus paper blue and phenolphthalein red Bases react with acids to produce a salt and water The pH level of bases ranges from 8 to 14 A base with a pH of 8 is a weak base A base with a pH of 14 is a strong base Examples of bases: baking soda, Tums, Drano, NaOH pH SCALE pH 7 - neutral Neutralization The reaction between an acid and a base is called neutralization Acids are a source of H+ ions, hydrogen ions Bases are a source of OH- ions, hydroxide ions During neutralization these two ions combine to form water The products of neutralization are ALWAYS a salt and water Example If we reacted hydrochloric acid and potassium hydroxide, neutralization would occur HCl + KOH KCl + H2O Our product is potassium chloride, which is a salt, and water ACIDS AND BASES EVERYWHERE!!!!!! Industrial processes: Acids and bases are used as reactants and catalysts to make goods Biological systems: Acids can be found throughout our body, our stomachs contain HCl, DNAdeoxyribonucleic acid, and amino acids which make up our proteins Bases react with the oil in our skin to form a soapy film Domestic applications: You have many acids and bases in your home that you use for a variety of reasons Acids: orange juice, soft drinks, vinegar Bases: bleach, oven cleaner, baking soda