* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture 32. Titan and its Atmosphere.
Survey
Document related concepts
Transcript
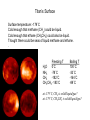
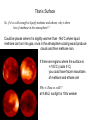
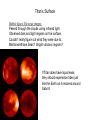
First Images from the Mars Reconnaisance Orbiter (MRO) Reached Mars on March 10 Now: turning elliptical orbit into a more circular orbit. First test images. Craters (fresh/old?) Gullies Dunes Begins collecting data in November. $720 million mission. ESA Venus Express Went into Orbit $260 million mission. 25th Anniversary of the First Shuttle Launch Reflection in the news media over the Crew Exploration Vehicle. -lack of funds to accomplish -costs over 1/5 of NASA’s budget -requires significant transformation in NASA Lecture 32. Titan and its Atmosphere. reading: Chapter 8 Titan Earth: 12,700 km Mars: 6,800 km Ganymede:5262 km Moon: 3476 km Largest Moon of Saturn - 5150 km. Discovered by Christiaan Huygens in 1655. Voyager 1 flyby in 1980. In 2004, Cassini orbiter made close observations. Jan 2005, Huygens probe landed on the surface. Composition: ~50% water ice and ~50% rock Similar composition as Ganymede & Callisto. Density of water: 1.0 g/cm3 Titan/Callisto: 1.9 g/cm3 Earth: 5.5 g/cm3 May have a rocky core and a mantle of ice. Rotates every 16 days, is in synchronous rotation. Voyager found southern hemisphere to be brighter, are seasonal differences in brightness. Titan’s Atmosphere Unique that it has a dense atmosphere of 1.6 bars and is mostly N2. 77-85% N2, 12-17% argon, 3-6% methane, 0.2% H2 Trace amounts of organic compounds: ethane, propane, hydrogen cyanide, CO2, CO, acetylene Organic compounds when methane undergoes photochemical QuickTime™ and a reactions from the faint sunlight. TIFF (Uncompressed) decompressor Produces a thick smog,arehazy neededatmosphere. to see this picture. Haze layer is ~300 km above the surface. There are distinct layers in the haze. Titan’s orbit is sometimes outside of Saturn’s magnetosphere. Solar wind particles may ionize the atmosphere and cause some of the photochemical reactions in the atmosphere. Thick atmosphere obscures the surface in visible light. false-color Voyager 1 image of haze layers Voyager Observations Atmosphere structure and dynamics determined by 2 instruments: -radio occultation How radio waves are scattered and bent as they pass through the atmosphere determine the T and density of the atmosphere -infrared spectroscopy Different compounds absorb heat energy at different wavelengths and vibrate. Measure how heat energy from sunlight is absorbed (produces peaks and valleys in the spectrum). Each compound has particular fingerprint. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Voyager Observations, cont. Voyager Observations, cont. large number of hydrocarbons and N-containing compounds Titan’s Surface Surface temperature: -179˚C Cold enough that methane (CH4) could be liquid. Cold enough that ethane (CH3CH3) could also be liquid. Thought there could be seas of liquid methane and ethane. . Freezing T H2O 0˚C NH3 -78˚C CH4 -182˚C CH3CH3 -183˚C Boiling T 100˚C -33˚C -164˚C -89˚C at -179˚C, CH4 is solid/liquid/gas? at -179˚C, CH3CH3 is solid/liquid/gas? Titan’s Surface So, if it is cold enough to liquify methane and ethane, why is there lots of methane in the atmosphere?? Could be places where it is slightly warmer than -164˚C where liquid methane can turn into gas, once in the atmosphere cooling would produce clouds and then methane rain. If there are regions where the surface is <-183˚C (cools 4˚C) you could have frozen mountains of methane and ethane ice! Why is Titan so cold?? at 9.6AU, sunlight is 100x weaker Titan’s Surface Hubble Space Telescope images: Peered through the clouds using infrared light. Observed dark and light regions on the surface. Couldn’t really figure out what they were due to. Methane/ethane Seas? Bright volcanic regions? If Titan does have liquid seas, they should experience tides just like the Earth as it revolves around Saturn! Clouds Scattered clouds. Composed of methane, ethane, or simple organic compounds. More complex organic compounds create the orange color. Clouds change hourly, presumably deposit methane rain onto the surface. Tholins Polymer formed from uv irradiation of CH4 or CH3CH3. Abundant on icy bodies in the solar system. Coined by Carl Sagan to describe unknown organic substances obtained in Miller-Urey-type experiments using gas mixtures found in Titan’s atmosphere. Produce a thick orange goo. When dissolved, they produce amino acids with both handedness. Polymers of C, H, and N. Over 75 different compounds in it. PAH family: benzene (1 ring), 2-4 rings cyclic compounds with C and N: includes bases found in nucleic acids How are Tholins Produced? UV light causes reactions to occur high in the atmosphere (photochemical reactions) Solid particles Tholins likely rain down to the surface. Speculation: could be km-deep deposits of tholins on the surface. Titan as a Model for Early Earth Methane-rich, N2-rich atmosphere, lack of oxygen. An early methane greenhouse helps solve Earth’s Faint Young Sun Problem. Photochemical reactions producing organic compounds. Some think there could have been similar hazes on early Earth. Hydrogen cyanide in the atmosphere - is a prebiotic compound in the abiotic synthesis of amino acids. Titan is soooo cold - chemical reactions proceed very sloooowly. So fewer number of organic compounds are produced. At -179˚C a chemical reaction will be 1042 times slower than at room temperature! Life on Titan? Do you have the 5 thing needed to have life? Properties of Water that are Important for Life It is the “universal solvent” because it can dissolve the most substances than any other liquid. Electrical charge differential helps it dissolve ions, like sodium chloride. Water molecules are attracted to each other creates surface tension Water forms drops, capillary action allows water to be sucked up plant roots and blood vessels Water is a polar compound. Water can also dissolve Ions are needed for life! uncharged organic compounds, like sugars. It is found in all three states solid, liquid, gas on the Earth Methane and Ethane Liquids Are hydrocarbons - so have a greasy nature. Are non-polar compounds so they: -don’t dissolve ions. -don’t have much surface tension. Lecture 33. Cassini-Huygens Mission. reading: Chapter 8