* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Polypeptide and protein structure
Vesicular monoamine transporter wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Ligand binding assay wikipedia , lookup
Proteolysis wikipedia , lookup
Point mutation wikipedia , lookup
Protein structure prediction wikipedia , lookup
Mineralized tissues wikipedia , lookup
Drug design wikipedia , lookup
Genetic code wikipedia , lookup
Metalloprotein wikipedia , lookup
Biochemistry wikipedia , lookup
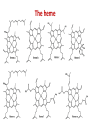
Structure-Function relationship Nafith Abu Tarboush DDS, MSc, PhD [email protected] www.facebook.com/natarboush The heme Myoglobin The first to be determined structurally A single polypeptide chain (153 a.a) A single heme in a hydrophobic pocket 8 -helices; no -sheets Most polar (exterior) Nonpolar (interior) Two His residues Fe(II) coordination Hemoglobin • A tetramer 22: -chains (141 a.a) & -chains (146 a.a) • 1 heme group in each (4O2) • Myoglobin (storage) vs. hemoglobin (transport): positive cooperativity & saturation percentage • lungs (100 torr), capillaries (20 torr) Conformational Changes Accompany Hb Function Oxygenated vs. deoxygenated (different crystal structures) Bohr effect: (tissues vs. lungs): lower pH → ↑H+→ protonation (ex. His146) → Asp94- His146 salt bridge → lower O2 affinity Oxy form is a stronger acid (less H+ affinity) than de-oxy (higher H+ affinity) CO2 (tissues vs. lungs) → H2CO3 → HCO-3+H+ → ↓ pH (less O2 affinity) O2 binding affinity, CO2 & H+ in myoglobin Conformational Changes Accompany Hb Function 2,3 BPG have an allosteric binding, lower affinity Hb F has a higher affinity for O2 due to: α2γ2 Less affinity to BPG (adult hemoglobin, βHis143-BPG salt bridge, fetal hemoglobin, the γ –Ser instead of His) The Collagen Triple Helix The most abundant protein in vertebrates Organized in water-insoluble fibers Have a great strength Consists of three polypeptide chains wrapped around each other in a ropelike twist, or triple helix Has a repeating sequence of the amino acids; X1—X2(Pro, ProOH)—Gly Hydroxy-lysine also occurs in collagen The triple helix (tropocollagen) is 300 nm long and 1.5 nm in diameter Held together by H-bonding Each strand have ≈800 amino acids (300KDa) Collagen, types & diseases Collagen I: skin, tendon, vessels, • • • bone Collagen II: cartilage Collagen III: reticular fibers, alongside type I Collagen IV: basement membrane Collagen V: cell surfaces, hair and placenta Cross-linked intra- & inter-molecularly Cross-linking amounts varies according to tissue & increases with age Deficiency of cross-linking (Scurvy & osteogenesis imperfecta) Keratin • Principal component of epidermis and related appendages (hair, horn, nails, & feathers) • α(mammals) or β(birds & reptiles) • Mammals: ≈30 types, tissue-specific • Structure: α-helix, coiled coil Elastin • Rich in hydrophobic amino acids (ex. Gly, Val & Pro); mobile hydrophobic regions bonded by crosslinks between Lys • Elastic fibers in arteries are composed mainly of elastin (≈70%) • Tropoelastin → Elastin (Lysyl oxidase) Elastin & hydroxylysine • Collagen contain lysine that can be hydroxylated by lysyl-hydroxylase to form hydroxyl-lysine or by lysyl-oxidase to form Allysine • Cross-linking of elastin occurs through the enzyme lysyl-oxidase producing the Allysine, the pathway for oxidation through lysylhydroxylase does not occur in elastin