* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lehninger Principles of Biochemistry 5/e
Survey
Document related concepts
Transcript
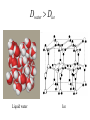
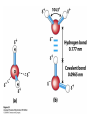
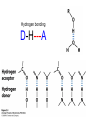
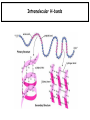
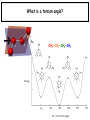
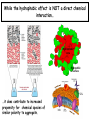
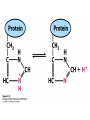
Chapter 2 1.) Properties of water, hydrogen bonding and the hydrophobic effect 2.) Other important intramolecular interactions 3.) Acid/base chemistry Dwater > Dice Liquid water Ice Hydrogen bonding D-H---A Water is a “locally structured transient gel.” While the conformational entropy (# of possible arrangements) of the lipid is decreased by sequestering it, the overall system entropy increases due to the dramatically increased number of ways that the HOH molecules can be arranged. This is a direct result of the transient nature of HB networks…meaning, they are continuously fluctuating and any number of HOH molecules can occur within a given ‘location.’ Diffusion is from high to low concentration! Chapter 2 1.) Properties of water, hydrogen bonding and the hydrophobic effect 2.) Other important intramolecular interactions 3.) Acid/base chemistry Intramolecular H-bonds Relative strength of the chemical interactions that we are interested in Covalent bonds Increasing strength Salt bridges (aka ionic bonds) Hydrogen bonds Dipole-dipole interactions van der Waals interactions (aka, London forces) Note that the hydrophobic effect is NOT a direct force between nuclei. Rather, it is a bulk colligative property arising from the overall number of degrees of freedom within the system (as already discussed). Notes Hooke’s law, U = 1/2k·l2 Hooke’s law, U = 1/2k·q2 We will ignore improper torsions Sinusoidal potential. Note the three minima, which depending on the local chemistry, may or may not be equally deep. U = q1q2 / (4pe·rij) Positive (destabilizing) values when ++ or --. Morse curve. Dissecting the force field ki ki 2 2 U(r ) = (li - li,o ) + (qi - qi,o ) + 2 2 bonds angles N å å Un (1+cos(nw - g )) + 2 torsions å æ éæ ç 4e êç s ij ç ij êçè rij j= i+1è ë åå i=1 ö ú+ qi q j ÷ ú 4 pe o rij ÷ û ø 6ù ö æs ö ij ÷÷ - çç ÷÷ ø è rij ø 12 What is a torsion angle? CH3-CH2-CH2-CH3 While the hydrophobic effect is NOT a direct chemical interaction… Hydrophobic core Hydrophilic surface …it does contribute to increased propensity for chemical species of similar polarity to aggregate. Chapter 2 1.) Properties of water, hydrogen bonding and the hydrophobic effect 2.) Other important intramolecular interactions 3.) Acid/base chemistry (alkaline) Consider the halide diatomic acids (HF, HCl, etc.) Q: Which are “strong” acids? Which are not? Why? Nomenclature of common acid/conjugate base pairs Amino acid sidechain pKa values* Q: Why is there an asterisk here? Residue pKa values: CT: 3.8 (R-CO2H) Asp: 4.0 (R-CO2H) Glu: 4.4 (R-CO2H) His: 6.5 (imidazole) NT: 8.0 (R-NH3+) Cys: 8.5 (R-SH) Tyr: 10.0 (Ph-OH) Lys: 10.0 (R-NH3+) Arg: 12.0 (guanidinium) The answer is yes! You must know these values.