* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document 8265878
Survey
Document related concepts
Pharmacognosy wikipedia , lookup
Orphan drug wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Drug design wikipedia , lookup
Drug interaction wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug discovery wikipedia , lookup
Transcript
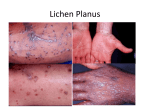
IL-‐36 proteoly6c processing Inhibitors Treatment for Inflamma-on Overview Applica,ons The technology describes strategies, as well as novel chemical en66es, for the inhibi6on of the proteoly6c processing and ac6va6on of members of the Interleukin-‐36 (IL-‐36) family of cytokines-‐ which play a role in promo6ng inflamma6on, especially inflamma6on of the skin as is observed in psoriasis. Individuals that carry loss-‐of-‐func6on muta6ons in the IL-‐36 receptor antagonist (IL-‐36RA) display a severe and highly debilita6ng form of psoriasis, called generalized pustular psoriasis. We have iden6fied the proteases that ac6vate IL-‐36alpha, IL-‐36beta and IL-‐36gamma and demonstrate that inhibi6on of these proteases (specifically elastase and cathepsin G) greatly aJenuates the biology ac6vity of IL-‐36 family cytokines. These novel small (<400daltons) polypep6de inhibitors of proteases have a therapeu6c poten6al for inflammatory condi6ons where excessive or deregulated IL-‐36 signalling may have a func6on in disease pathology, such as psoriasis. Specifically, direct therapeu6c u6lity as inhibitors of neutrophil granule protreases (Cathepsin G, Elastase and proteinase 3) in inflammatory diseases. The applica6on of small molecule inhibitors directly onto affected areas of skin circumvents a major disadvantage of current therapies for psoriasis and related skin diseases, namely the systemic delivery of potent immunosuppressive drugs or neutralizing an6bodies that could make pa6ents vulnerable to sporadic infec6ons. Market Therapeu6cs: Synthesis, Formula6on, Processing and Drug Delivery IP Status Priority Applica6on filed EP14185397.8 Psoriasis is a chronic skin disease that affects over 100,000 people in Ireland alone and is es6mated to affect 2-‐4% of the popula6on in western countries. Market reports predict that the world psoriasis drug market will be worth $8.9bn in 2018. Those products, trea6ng that indica6on generated £6bn in 2012 according to ‘Psoriasis Treatment: World Drug report 2013-‐2023’ IL-36 gamma Technology Following on from this discovery, specific novel polypep6de en6tles have been developed that are capable of blocking the ac6va6on of IL-‐36 cytokines by cathepsin G and elastase; Glu-‐Pro-‐Phe-‐CMK; Ala-‐Phe-‐Leu-‐Phe-‐CMK; Lys-‐Ala-‐ Leu-‐CMK, Arg-‐Ala-‐Val, Asp-‐Thr-‐Glu-‐Phe, Ala-‐Pro-‐ Leu, Pro-‐Gln-‐Arg, Arg-‐Pro-‐Leu and deriva6ves thereof are capable of inhibi6ng the processing and ac6va6on of IL-‐36 family cytokines by neutrophil granule proteases Opportunity Research collabora6on, Available to license Researcher(s) Prof. Seamus Martain Technology and Patent Status A priority patent had been filed with the European patent office; EP14185397.8 Contact Dr. Emily Vereker, Case Manager, Life Sciences The opportunity Trinity College is seeking to collaborate and/or licence the technology to an pharmaceu6cal company for development and commercialisa6on. [email protected] +353 1 896 4152 Reference: SM01-‐498-‐01