* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Plateau principle wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Orphan drug wikipedia , lookup
Compounding wikipedia , lookup
Psychopharmacology wikipedia , lookup
Drug design wikipedia , lookup
Theralizumab wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacognosy wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug discovery wikipedia , lookup
Prescription costs wikipedia , lookup
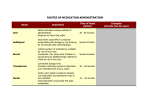
Pharmacology Introduction PHARMACOLOGY Greek Word Pharmacon Drug Logos Science • Pharmacology: the science that deals with drugs. • Drug: a substance that is used for prevention, treatment & diagnosis. Names Drugs Drug of Names Chemical name • The drug’s chemical composition and molecular structure… is the name by which the chemist knows it. Generic name (nonproprietary name, Official) • Name given by international organization of drug nomenclature & independent of the manufacture. Trade name (proprietary name, brand name) • The drug has a registered trademark; use of the name restricted by the drug’s owner (usually the manufacturer) • Pharmacologic Principles: Drug Names • Chemical Name : N-(4-Hydroxyphenyl)acetamide • Generic Name: Acetaminophen. Paracetamol. • Trade Name: Panadol® Revanin® Pandarin® Source of drugs 1. Plants: such as digitalis, vincristine. 2. Animals: insulin 1. Minerals: as iron, iodine and zinc 3. Microorganism: antibiotics 4. Synthetic and chemical substance: as sodium bicarbonate. 5. Drugs produced by genetic engineering : Human insulin, human growth hormone Drug classification • • • • Drugs may be classified by: Their effect on particular Body system. Their Therapeutic uses. Their chemical structure. • E.g: morphine can be classified as central nervous system depressant, a narcotic or opioid analgesic, & as an opiate( derived from opium) • Prescription & non prescription drugs American consumers have 2 routes of access to therapeutic drugs: 1.Over the counter (OTC) purchase of drugs that do not require a prescription. 2. By prescription or order from a licensed health care provider. • Using prescription drugs for non therapeutic purposes by persons who are not authorized to have the drugs or whom they are not prescribed , is illegal. Pharmacokinetic Processes The study of what the body does to the drug: “ADME” is key Absorption Distribution Metabolism Excretion • What is the effect of the body on the drug? Pharmacologic Principles Pharmacodynamics • The study of what the drug does to the body: – The mechanism of drug actions in living tissues Dosage Plasma Site of Concen. Action Pharmacokinetics Effects Pharmacodynamics • Routes of administration • Selection of the best route of drug administration depends on: • Patient condition • Drug property Definition: A route of administration is the path by which a drug, fluid, poison or other substance is brought into contact with the body. CLASSIFICATION Enteral Oral Sublingual Rectal Parenteral Injections Intravenous Intramuscular Subcutaneous Intra-arterial Intra-articular Intrathecal Intradermal Others LOCAL •Skin topical •Intranasal •Ocular drops •Mucosal-throat, vagina, mouth, ear Inhalational Transdermal Enteral Routes • Enteral - drug placed directly in the GI tract: – sublingual - placed under the tongue – oral - swallowing (p.o.) – rectum - Absorption through the rectum Oral Route • Advantages: Convenient- portable no pain easy to take Cheap – no sterilization no expert. Oral Routes • Disadvantages: Variable bioavailability Food can affect absorption Local effect: taste,irritation Unsuitable for Unconscious pts. First pass effect First pass effect • Drugs absorbed orally are transported to liver by Portal circulation before reach systemic circulation, thus are extensively metabolized in the Liver, this is known as first pass effect. Swallowed Drug Digestive system Hepatic portal system Liver Rest of the body Dosage forms Capsules, powders Tablets Syrup, Suspension, elixirs Tablets Hard- gelatin capsule Syrup Soft- gelatin capsule Sublingual Medication Administration Place the pill between the underside of the tongue and the floor of the oral cavity. Sublingual • Advantages: No first pass effect Rapid absorption and effect • Disadvantages: Inconvenient Small doses only. Bitter tasting can not give to unconscious patient. Buccal/Sublingual route (Cont.) Applicable dosage forms: Tablets Chewable tablets 3. Rectal Drugs are administered rectally for their local or for systemic effect Advantages: • Reduced first pass hepatic effect • Useful if the drug induces vomiting when given orally or if the patient is already vomiting Disadvantages: • Absorption is often incomplete • Chance for local irritation • Loss of dosage form by defecation • • • • Applicable dosage forms: Suppositories Ointments Solutions, usually employed as enemas Prepackaged enema container • • • • B. Parentral Route A drug administered parentrally is one injected via a hollow needle into the body at various sites and to varying depth. Parentral administration is used for drugs that are: Poorly absorbed from GIT Unstable in the GIT Parenteral Routes – Intravascular (IV, IA)- placing a drug directly into the blood stream – Intramuscular (IM) - drug injected into skeletal muscle – Subcutaneous - Absorption of drugs from the subcutaneous tissues. Parenteral route 1. Intravenous (I.V.) I.V. injection is the most common parentral route Drugs may be given into peripheral vein over 1-2 min or longer by infusion Advantages: • With I.V. administration the drug avoids the GIT, so bypass the Liver • Large volume can be given • Used to treat acute cases as epileptic seizures, cardiac arrhythmias Disadvantages: • Unlike drugs present in GIT, those that are injected cannot be recalled by strategies such as emesis. • Chance of infection • More expensive. • Require trained personnel for their proper administration Parenteral route (Cont) B-Intramuscular : (into the skeletal muscle). suitable for injection of drug in aqueous solution (rapid action) and drug in suspension (sustained release). Large volume can be given 1-5 ml Should be given at an angle of 90˚ Disadvantages: • Trained personnel required for injection • Painful • Absorption is sometimes erratic 3.Subcutaneous (S.C.) • This involves administration of a dose into the fatty layer just under the skin • The needle is inserted at an angle of 45 ْ Advantages: • Can be given by the patient. Disadvantages: • Can be painful • Irritant drugs can cause local tissue damage • Intrathecal/intraventricular: It is sometimes necessary to introduce drugs directly into the cerebrospinal fluid. For example, amphotericin B is used in treating cryptococcal meningitis Intradermal • Intradermal injections are delivered into the dermis, or the skin layer underneath the epidermis at angle 10-15˚. Mainly used for testing sensitivity to drugs. the position of needle for I.M, S.C, Intradermal injection Ampules and Vials Vials Ampules Others- Topical route: Topical: Drugs are applied topically to the skin or mucous membranes, mainly for local action. I Skin cream, ointment (local action) Lotion, paste,gel II Mucosal membranes • eye drops (onto the conjunctiva) • ear drops • intranasal route (into the nose) Others- Transdermal Transdermal - absorption of drug through skin (systemic action) stable blood levels no first pass metabolism. Inhalation route: - Used for gaseous and volatile agents and aerosols. • Delivery of drugs across the large surface area of mucous membranes of respiratory tract. • Local effect – Bronchodilator • Systemic effect – General anesthesia • Advantages: - Rapid - No first pass effect • Disadvantages: - Irritation - Some particles may be precipitated in mouth or throat Nebulizer Inhaler DRUG DOSAGE FORMS Tablets Capsule Aerosol Injection Suspension Infusion Cream Solution Absorption • The passage of drug from site of administration (from out side) to the blood stream. • To do so the drug has to pass through cell membranes. • Cell membranes are of lipid bilayer and pores. • Most drugs are absorbed by “Passive diffusion” or simple diffusion. III. ABSORPTION OF DRUGS • A. Transport of Drug from the GIT 1. PASSIVE DIFFUSION - The driving force for passive absorption of a drug is the concentration gradient - Does not involve a carrier - Vast majority of drugs gain access to the body by this mechanism Mechanisms of Passage Across Membranes ACTIVE TRANSPORT ♦ requires carrier ♦ energy consuming ♦ against a concentration gradient ♦ saturability ♦ selectivity • Factors influencing absorption: 1. Effect of pH on Drug Absorption • Most drugs are either weak acids or weak bases which disassociate in the body fluid • Diffusion of the non-ionized form of a weak acid through a lipid membrane. B. Diffusion of the nonionized form of a weak base through a lipid membrane . Environmental pH and Ionization If we put an acidic drug in an environment with a lot of H+ (low pH) what will this equilibrium do? HA HA HA H+ + A- Equilibrium System H+ fromatacid environment Non-ionized form predominates! Acidic drugs are best absorbed from acidic environments Basic drugs are best absorbed from basic environments Factors influencing absorption: 2. Blood flow 3. Contact time at absorption site 4. Total surface area available for absorption Bioavailability Definition: the fraction of the administered dose reaching the systemic circulation unchanged for i.v.: 100% for non i.v.: ranges from 0 to 100% e.g. lidocaine bioavailability 35% due to destruction in gastric acid and liver metabolism Factors that influence bioavailability: • First-pass hepatic metabolism • Solubility of the drug • Chemical instability • Drug Distribution refers to the movement of a drug from the blood to various tissues of the body and depend on: 1.Blood flow. 2. Capillary permeability 2. Capillary permeability Membranes • Special barriers to distribution • Blood/Brain Barrier: This barrier provides a protective environment for the brain. Speed of transport across this barrier is limited by the lipid solubility of the drug and its molecular weight… • Placental Barrier: This barrier separates two distinct human beings but is very permeable to small molecular weight drugs. Blood-Brain Barrier The blood brain barrier consists of cell tightly packed around the capillaries of the CNS. What characteristics must a drug possess to easily cross this barrier? Non-protein bound, non-ionized, and highly lipid soluble Why? 3. Binding of drugs to proteins • D + plasma protein [D-plasma protein] complex Reversible binding to plasma proteins sequesters drugs in a non-diffusible form & slows their transfer out the vascular compartment Plasma albumin is the major drug-binding protein & act as a drug reservoir LOCUS OF ACTION “RECEPTORS” Bound Free ABSORPTION TISSUE RESERVOIRS Free Bound Free Drug Bound Drug SYSTEMIC CIRCULATION BIOTRANSFORMATION EXCRETION Volume of Distribution(Vd) ♦ It is the volume that would be required to contain all the drug in the body at the same concentration as in the plasma ♦ drugs with large volume of distribution will have a longer half-life and duration of action Elimination of drugs from the body M A J O R KIDNEY LIVER filtration secretion metabolism secretion M I N O R LUNGS OTHERS exhalation mother's milk sweat, saliva etc. (reabsorption) VII. DRUG METABOLISM Drugs are often eliminated by biotransformation and or excretion into the URINE OR BILE. LIVER – the MAJOR SITE FOR DRUG METABOLISM • Renal elimination of a drug • Effect of drug metabolism On reabsorption in the distal tubule. Reactions of Drug Metabolism The kidney cannot efficiently eliminate lipophilic drugs , therefore lipid soluble agents must 1st be metabolized in the liver using 2 general sets of reactions PHASE I PHASE II Phase I • uses the P 450 system Located within the endoplasmic reticulum of hepatocytes. • Its function is to convert lipophilic molecules into more polar (H2Osoluble) molecules by introducing a polar • Phase I metabolism may group as –OH2 –NH2 increase, decrease, or • Hydrolysis leave unaltered the drug’s pharmacologic • Oxidation activity. • Reduction Phase II • Consists of conjugation reactions • Uses substrates like glucuronic acid, or an amino acid • Renders the metabolites INACTIVE and more water soluble • The highly polar drug conjugates may then be excreted by the kidney or bile • Factors Affecting Drug Metabolism • P450 activity is genetically determined: – Some persons lack such activity leads to higher drug plasma levels (adverse actions) – Some persons have high levels leads to lower plasma levels (and reduced drug action) • Other drugs can interact with the P450 systems – Either induce activity. e.g : rifampicin – Inactivate an enzyme system. e.g: cimetidine • Age, neonates and very old. • Pathological factors – Liver diseases. Question • The addition of glucuronic acid to a drug: A. Decreases its water solubility. B. Usually leads to inactivation of the drug. C. Is an example of a Phase I reaction. D. Occurs at the same rate in adults and newborns. E. Involves cytochrome P450. Elimination by the Kidney • Excretion - major 1) glomerular filtration 2) tubular reabsorption/secretion • Proximal tubular secretion • Distal tubular reabsorption Clearance ♦ the measure of the ability of the body to eliminate the drug ♦ the sum of hepatic metabolism and renal excretion • The speed of elimination is the main factor in deciding the duration of action of the drug & is referred plasma half-life (t½) of the drug T½: The time required to change the plasma concentration of the drug by one half Half- life (T½) ♦ often used to determine frequency of administration ♦ determines the time to attain steady-state concentration • Factors influencing the dosage regime • The blood level & therapeutic effectiveness depend on the dose, distribution within the body & speed of elimination. • Rapidly excreted drugs with short t½ will require frequent administration to maintain a fairly constant concentration in the body where as those that are eliminated slowly can be given once or twice • With repeated dosing, concentration in plasma climbs until more or less steady state (SS) is obtained. • Steady-state occurs after a drug has been given for approximately five elimination halflives. Steady State ♦ the point where rate of drug availability equals rate of elimination ♦ constant drug concentration ♦ point where expect maximum drug effect ♦ usually attained after 4-5 half lives • To hasten achievement of SS & full therapeutic effect with more slowly excreted drugs, a large loading dose is given followed by smaller maintenance doses Single dose – Loading dose 7 Plasma Concentration 6 Therapeutic level 5 4 3 Repeated doses – Maintenance dose 2 1 0 0 1.9 3.9 5.9 7.9 9.9 11.9 13.9 15.9 17.9 19.9 21.9 23.9 Time Dose Response Relationships • Loading dose – Bolus of drug given initially to rapidly reach therapeutic levels • Maintenance dose – Lower dose of drug given continuously or at regular intervals to maintain therapeutic levels • Pharmacodynamics • The action of the drug on the body • The drug interacts with a receptor: • D+R D – R complex effect • This reaction is reversible & in order to occur, an affinity between the drug & receptor Non-receptor Mechanisms • Actions on Enzymes – Drugs altering enzyme activity alter processes catalyzed by the enzymes – Examples • Cholinesterase inhibitors • Monoamine oxidase inhibitors Non-receptor Mechanisms • Changing Physical Properties – E.g:Mannitol – Changes osmotic balance across membranes – Causes urine production (osmotic diuresis) Non-receptor Mechanisms • Changing Cell Membrane Permeability – Lidocaine • Blocks sodium channels – Verapamil, nefedipine • Block calcium channels Non-receptor Mechanisms • Combining With Other Chemicals – Antacids – Chelation of heavy metals. • Replacement therapy as hormones& vitamins Non-receptor Mechanisms • Anti-metabolites – Enter biochemical reactions in place of normal substrate “competitors” – Result in biologically inactive product – Examples • Some anti-neoplastics • Some anti-infectives • Types of drugs: • Agonist: a drug that interact with a receptor followed by effect • Antagonist: a drug that interact with a receptor but with no effect • Partial Agonist: a drug that bind to a receptor followed by weak effect PARTIAL AGONISTS - EFFICACY Even though drugs may occupy the same # of receptors, the magnitude of their effects may differ. % Maximal Effect 1.0 0.8 0.6 0.4 0.2 0.0 0.01 0.10 1.00 [D] 10.00 (concentration units) 100.00 1000.00 Receptor Interactions Lock and key mechanism Agonist Receptor Agonist-Receptor Interaction Receptor Interactions Competitive Inhibition Antagonist Receptor DENIED! Antagonist-Receptor Complex Receptor Interactions Non-competitive Inhibition Agonist Antagonist Receptor DENIED! ‘Inhibited’-Receptor • Dose – Response curve Graded -dose response: • Drugs A & B have the similar efficacy but they differ in potency • Efficacy: The maximal response produced by the drug • Potency: How much of the drug required to produce a response • More potent drug is capable to produce the desired effect with smaller dose • In the case of drug C it has lower efficacy than A & B & lower potency • Therapeutic Index The ratio of the dose that produce toxicity to that produce clinical response Therapeutic index TD50/ED50 • TD50: The dose that cause toxicity in 50% of individuals • ED50: The dose that produce an effect in 50% of individuals The ratio must be large in order to be a safe drug