* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CONVULSIVE TAILS
Psychedelic therapy wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Psychopharmacology wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Neuropharmacology wikipedia , lookup
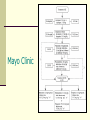
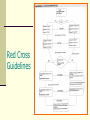
STATUS EPILEPTICUS UPDATE Jo Wilmshurst Department of Paediatric Neurology Red Cross Children’s Hospital What are the protocols? How should we monitor these children? Definitions Status epilepticus: Generalised convulsions > 30 minutes = brain damage / neuronal cell death Refractory status: Generalised convulsions > 1hour, resistant to level 1-2/3 intervention – i.e. need PICU intervention The longer it takes to gain control the worse the outcome and the harder it will be to terminate Sz Outcome influenced by underlying aetiology – encephalitis worst result Scott et al, ARCH 1998 Holtkamp et al; JNNP 2005 Causes Fever Medication change Unknown Metabolic Congenital Anoxic Other (trauma, vascular, infection, tumour, drugs) 36% 20% 9% 8% 7% 5% 15% Haafiz et al; Ped Emerg Care 1999 Mortality Adults Children 15-22% 3-32% No figures for SA Fountain et al; Epilepsia 2000 Lacroix et al; CCM 1994 Sahin et al; Epilepsia 2001 Optimal intervention times Children > 5 years : typical GTCS seizure duration < 5 minutes Younger children and infants: paucity of data. Suggested time frame for a typical GTCS is less than 10-15 minutes. Mean age for status in children 3.4 years Lowenstein DH, Bleck T, Macdonald RL. Epilepsia 1999;40(1):120-2 Singh et al 2010 Neurology Diagnostic assessment of the child with status epilepticus Blood glucose Anti-epileptic drug (AED) levels. Toxicology testing Blood cultures Lumbar puncture (as clinically indicated & all children < 18 months) Neuroimaging: Insufficient evidence for routine neuroimaging (8% yield) Indications: When convulsive status is unexplained the patient remains unconscious, or new focal neurological signs become apparent. Evidence- based quideline American Academy of Neurology (ANN) and Child Neurology Society (CNS) Brain Monitoring Continuous Non-invasive Highly sensitive to a variety of brain insults Reasonably specific User friendly Not too expensive! Kurtz et al Curr Opin Crit Care 2009 Monitoring cEEG (continuous EEG – full head montage) The Gold standard – not viable in most SA settings Non-convulsive seizures Ischaemia aEEG (Amplitude-integrated EEG) Assessing if burst suppression attained Non-convulsive seizures Potential artefact Need to remember overall underlying cause usually the defining feature for the outcome of the child. The future? Basic external monitoring (BP, sats, HR) often underestimates true cerebral function Cerebral Near-infrared spectroscopy (cNIRS) Non-invasive Used as a tool to assess regional brain saturations (RSO2) Available in SA! Comparison studies with serological markers (S100beta and NSE) – performed well (better infact) Subbaswamy et al Neurocrit Care 2009 Treatment of Status Epilepticus Pre hospital treatment A&E treatment In-hospital treatment (Ward/High care) Anaesthesia (ICU) What recommendations exist for managing Status Epilepticus in Children? Mayo Clinic Boston Children’s hospital European expert opinion 2007 European expert opinion 2007 Children’s Hospital of Philadelphia Red Cross Guidelines APLS guidelines (2005; The convulsing child) ABCD ↓ (Level 1) Lorazepam IV/IO or diazepam pr / midazolam buccal ↓ Lorazepam IV / IO ↓ Paraldehyde pr (Level 2) ↓ Phenytoin IV / IO / Phenobarbitone IV / IO ↓ (Level 3) RSI with Thiopental Level one Arrival – First Hosp intervention Benzodiazepine Diazepam PR/IV/IO Midazolam IN/SL/IV/IO Lorazepam IV/PR/IO Repeat if necessary Good specialist consistency, good study data Scott et al;Lancet 1999 Jeannet et al;Europ J Paed Neurol 1999 DeNegri et al; Pediatr Drugs 2001 Diazepam versus Lorazepam Both are equally effective at aborting status epilepticus. IV lorazepam vs IV diazepam Rectal lorazepam might be more effective than rectal diazepam Lorazepam: Substantial longer duration of anti-seizure activity (lipidsoluble) Less seizure recurrence and fever repeat doses required. Appleton R et al Cochrane Database Syst Rev 2008 Jul 16;(3) Transmucosal pharmacological therapy Intranasal midazolam as effective as intravenous diazepam Buccal midazolam as effective as rectal diazepam. Intravenous formulations of midazolam (given buccal or intranasal routes) are relatively inexpensive. Caregivers prefer intranasal midazolam to rectal diazepam. Appleton R et al Cochrane Database Syst Rev 2008 Jul 16;(3) Paraldehyde Treatment with IV phenytoin as a second-line therapy was associated with a 9-times greater likelihood of seizure termination than was treatment with paraldehyde Chin R, Neville B et al Lancet Neurol 2008;7:696-703 Level 2 intervention Phenytoin IV over 20 mins, cardiac monitor, large vein, not mixed with glucose Phenobarbitone IV/IM Push, flush through, monitor for resp depression and hypotension Both agents fairly accepted BUT studies becoming more limited small numbers less children Shanner et al;Neurol 1988 Prasad et al;Ann Neurol 2002 Phenytoin Takes 30 minutes to administer Requires a syringe driver Requires a large IV (NOT central) line Requires cardiac monitoring for potential cardiac toxicity Can only be given by IV route Cannot be repeated It not as effective as phenobarbitone DeToledo & Ramsay; Drug Saf 2000 Trieman et al; NEJM 1998 Fosphenytoin More favourable vehicle that does not contain proylene glycol and pH 8.6-9 Administer in dextrose containing IV solutions at a more rapid rate. Equally effective: Time for conversion of pro-drug to active drug (8-15minutes) = therapeutic phenytoin concentrations reached at the same time. Cost: Fosphenytoin 3 times equivalent dose of IV Phenytoin. Benefit: More favorable side effect profile (purple glove syndrome) Experimental Rescue therapy NG phenobarbitone 20mg/kg given during level 2 intervention Provided good airway protection and ability for gastric absorption … Study at Red Cross (Wilmshurst et al J Paed Child Health 2009) Any child / infant entering established status (Level 2) 1-4 hours to therapeutic levels SAFE No need for repeat dosage for therapeutic levels but for control of seizures could safely repeat Good viable addition to the protocol – especially where parenteral access or supply lacking Syed et al;Dev Pharmacol Ther 1986 Yska et al;Pharm World Sci 2000 Yukuwa et al;J Clin Pharm Ther 2005 Level 3 intervention Basically heading into refractory status Disastrous situation Resistant seizures – prob exacerbated by underlying cause ( eg encephalitis), secondary complications from drugs hypotension, respiratory depression all affecting brain perfusion Sahin et al;Neurol 2003 Scott et al, ARCH 1998 Holtkamp et al; JNNP 2005 Level 3 intervention: Treatment of refractory SE No prospective randomised trials comparing the effects of anesthetics in the treatment of RSE. Safety data lacking. Options: Barbiturate anesthetics: Pentobarbital (US) Thiopental (Europe Aus) Propofol Midazolam. Evidence based medicine: No recommendations on data available. Even in a large survey of neurologists in USA – little consensus for 3rd / 4th line intervention (J Neurol Sci 2003) Rosenow et al;Epileptic Disord 2002 Midazolam infusion Requires a syringe driver Greater risk of airway suppression (especially following previous Benzo boluses) Takes long time to gain control (range 15 mins – 4.5 hours) Potential for children left with prolonged seizures and irreversible neuronal cell death in centres without high care facilities NOTE: Excluded from APLS guidelines Rivera et al; CCM 1993 Lal Koul et al; ARCH 1997 Ozdemir et al; Seizure 2005 CLONAZEPAM INFUSION NO EVIDENCE Thiopentone Poor anticonvulsant Marked haemodynamic effects Prolonged drug effects if infusion used Local ICU capacity limited Staffing Monitoring Anaesthetic experience Very-high-dose Phenobarbitone Both barbiturates and benzodiazepines exert a primary effect on the GABA receptor complex. No antiepileptic ceiling effect ! No maximum dose beyond which further doses are likely to be ineffective >200mgkg! Complications: Sedative and respiratory-depressant properties more likely in combination with benzodiazepines. Hypotension unusual and related to the highest Phenobarbitone levels and easily controllable. Complications usually related to underlying aetiology Crawford et al; Neurol 1988 Intravenous Sodium Valproate FDA approved 1996. Not in APLS guidelines No reports of respiratory depression or hypotension. Caution in children with underlying liver disease or suspected mitochondrial disorder. Potential hepatic encephalopathy Comparative studies: Intravenous Sodium Valproate vs Diazepam infusion Intravenous Sodium Valproate vs Phenytoin. No large studies measuring efficacy Larger paediatric focused studies are needed Still need syringe driver Very expensive Drug of choice: Absence status Limdi et al; Neurology 2005 Rossetti & Bromfield; Neurology 2005 Limbdi N et al Epilepsia 2007 48(3):478-483 Morton L et al Pediatr Neurol 2007;36:81-83 Metha V et al J Child Neuro 2007; 22:1191 IV Levetiracetam FDA approved adults over 16 yrs since 2006 Limited data in children (most retrospective case reviews – n=10 and n=32) Loaded with 25-50mg/kg at level 3 Effective Safe Larger comparison studies needed Kirmani et al Ped Neurol 2009 Abend et al Pediatr Crit Care Med 2009 Gamez-Leyva et al CND Drugs 2009 Mx of status epilepticus in SA Most centres policy of repeated IV PB boluses Resulted (anecdotally) dramatic reduction in admissions to PICU and complications of status epilepticus IV Pb: WHO / IMCI guidelines first line for neonates; 2nd line for infants / children in Mx status Why is IV phenobarbitone so good for resource poor countries? Highly effective at controlling status Safe Cheap It can be given by rapid IV bolus It can be repeated It can be given by IM route No need for syringe driver If control not attained at 1 hour time to arrange transfer to tertiary unit – exceptional situation Crawford et al; Neurol 1988; Wilmshurst & Newton; DMCN 2005 Lee et al;Pediatr Neurol 2005 Overall Still do not have the ideal solution Still do not know what this is Need effective, rapidly acting, easy to administer, cheap agent .. Watch this space! Prospective comparison study underway relevant for RLC Final recommendations 2 targets Rapid identification of the underlying aetiology Affects treatment Affects prognosis Early initiation towards terminating SE Decreases morbidity and mortality Recommend Level 1 – benzodiazepines Level 2 – phenytoin, phenobarbitone, sodium valproate Level 3 – “other medications” e.g. levetiracetam and pharmacologic coma induction Abend and Marsh. Curr Treat Options Neurol 2009