* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Drug Discovery and Development
Polysubstance dependence wikipedia , lookup
Compounding wikipedia , lookup
Pharmaceutical marketing wikipedia , lookup
Psychopharmacology wikipedia , lookup
Development of analogs of thalidomide wikipedia , lookup
Orphan drug wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Theralizumab wikipedia , lookup
Pharmacognosy wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Drug interaction wikipedia , lookup
Prescription costs wikipedia , lookup
Drug design wikipedia , lookup
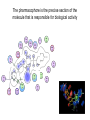
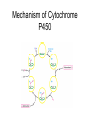
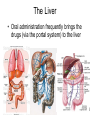
Drug Discovery and Development How are drugs discovered and developed? Basic Steps • Choose a disease • Choose a drug target • Identify a “bioassay” bioassay = A test used to determine biological activity. • Find a “lead compound” “lead compound” = structure that has some activity against the chosen target, but not yet good enough to be the drug itself. • If not known, determine the structure of the “lead compound” • Synthesize analogs of the lead • Identify Structure-Activity-Relationships (SAR’s) • Structure-Activity-Relationship (SAR) = How does the activity change as structure is systematically altered? • Identify the “pharmacophore” pharmacophore = the structural features directly responsible for activity • Optimize structure to improve interactions with target • Determine toxicity and efficacy in animal models. • Determine pharmacodynamics and pharmacokinetics of the drug. • Pharmacodynamics explores what a drug does to the body, whereas pharmacokinetics explores what the body does to the drug. Basic steps (cont.) • • • • • • Patent the drug Study drug metabolism Test for toxicity Design a manufacturing process Carry out clinical trials Market the drug Choosing a Disease • Pharmaceutical companies are commercial enterprises • Pharmaceutical companies will, therefore, tend to avoid products with a small market (i.e. a disease which only affects a small subset of the population) Choosing a Disease • Pharmaceutical companies will also avoid products that would be consumed by individuals of lower economic status (i.e. a disease which only affects third world countries) Choosing a Disease (cont.) • Most research is carried out on diseases which afflict “first world” countries: (e.g. cancer, cardiovascular diseases, depression, diabetes, flu, migraine, obesity). The Orphan Drug Act • The Orphan Drug Act of 1983 was passed to encourage pharmaceutical companies to develop drugs to treat diseases which affect fewer than 200,000 people in the US • Under this law, companies who develop such a drug are entitled to market it without competition for seven years. • This is considered a significant benefit, since the standards for patent protection are much more stringent. Identifying a Drug Target • Drug Target = specific macromolecule, or biological system, which the drug will interact with • Sometimes this can happen through incidental observation… Identifying a Drug Target (cont.) • Example: In addition to their being able to inhibit the uptake of noradrenaline, the older tricyclic antidepressants were observed to “incidentally” inhibit serotonin uptake. Thus, it was decided to prepare molecules which could specifically inhibit serotonin uptake. It wasn’t clear that this would work, but it eventually resulted in the production of fluoxetine (Prozac). HO NH2 N N CH3 H3C Imipramine (a classical tricyclic antidepressant) N H serotonin F3C HN O prozac The mapping of the human genome should help! • In the past, many medicines (and lead compounds) were isolated from plant sources. • Since plants did not evolve with human beings in mind, the fact that they posses chemicals which results in effects on humans is incidental. • Having the genetic code for the production of an enzyme or a receptor may enable us to overexpress that protein and determine its structure and biological function. If it is deemed important to the disease process, inhibitors (of enzymes), or antagonists or agonists of the receptors can be prepared through a process called rational drug design. Simultaneously, Chemistry is Improving! • This is necessary, since, ultimately, plants and natural sources are not likely to provide the cures to all diseases. • In a process called “combinatorial chemistry” large numbers of compounds can be prepared at one time. • The efficiency of synthetic chemical transformations is improving. Selectivity is Important! • e.g. targeting a bacterial enzyme, which is not present in mammals, or which has significant structural differences from the corresponding enzyme in mammals The Standards are Being Raised • More is known about the biological chemistry of living systems • For example: Targeting one subtype of receptor may enable the pharmaceutical chemist to avoid potentially troublesome side effects. Problems can arise • Example: The chosen target, may over time, lose its sensitivity to the drug • Example: The penicillin-binding-protein (PBP) known to the the primary target of penicillin in the bacterial species Staphylococcus aureus has evolved a mutant form that no longer recognizes penicillin. Choosing the Bioassay • Definitions: – In vitro: In an artificial environment, as in a test tube or culture media – In vivo: In the living body, referring to tests conductedin living animals – Ex vivo: Usually refers to doing the test on a tissue taken from a living organism. Choosing the Bioassay (cont.) In vitro testing • Has advantages in terms of speed and requires relatively small amounts of compound • Speed may be increased to the point where it is possible to analyze several hundred compounds in a single day (high throughput screening) • Results may not translate to living animals Choosing the Bioassay (cont.) In vivo tests • More expensive • May cause suffering to animals • Results may be clouded by interference with other biological systems Finding the Lead Screening Natural Products • Plants, microbes, the marine world, and animals, all provide a rich source of structurally complex natural products. • It is necessary to have a quick assay for the desired biological activity and to be able to separate the bioactive compound from the other inactive substances • Lastly, a structural determination will need to be made Finding the Lead (cont.) Screening synthetic banks • Pharmaceutical companies have prepared thousands of compounds • These are stored (in the freezer!), cataloged and screened on new targets as these new targets are identified Finding the Lead (cont.) Using Someone Else’s Lead • Design structure which is similar to existing lead, but different enough to avoid patent restrictions. • Sometimes this can lead to dramatic improvements in biological activity and pharmacokinetic profile. (e.g. modern penicillins are much better drugs than original discovery). Finding the Lead (cont.) Enhance a side effect O H2N S NH2 O sulphanilamide (an antibacterial with the side effect of lowering glucose levels in the blood and also diuretic activity) N Cl O O S NH O NH tolbutamide (a compound which has been optimized to only lower blood glucose levels. Useful in the treatment of Type II diabetes.) O H2N NH S S O O O Chlorothiazide (a compound which has been optimized to only display diuretic activity.) Use structural similarity to a natural ligand NH2 N(CH3)2 H N HO H3C S O N H 5-Hydroxytryptamine (5-HT) Serotonin (a natural neurotransmitter synthesized in certain neurons in the CNS) O N H Sumatriptan (Imitrex) Used to treat migrain headaches known to be a 5-HT1 agonist Finding the Lead (cont.) Computer-Assisted Drug Design • If one knows the precise molecular structure of the target (enzyme or receptor), then one can use a computer to design a perfectly-fitting ligand. • Drawbacks: Most commercially available programs do not allow conformational movement in the target (as the ligand is being designed and/or docked into the active site). Thus, most programs are somewhat inaccurate representations of reality. Finding a Lead (cont.) Serendipity: a chance occurrence • Must be accompanied by an experimentalist who understands the “big picture” (and is not solely focused on his/her immediate research goal), who has an open mind toward unexpected results, and who has the ability to use deductive logic in the explanation of such results. • Example: Penicillin discovery • Example: development of Viagra to treat erectile dysfunction Finding a Lead (cont.) Sildenafil (compound UK-92,480) was synthesized by a group of pharmaceutical chemists working at Pfizer's Sandwich, Kent research facility in England. It was initially studied for use in hypertension (high blood pressure) and angina pectoris (a form of ischaemic cardiovascular disease). Phase I clinical trials under the direction of Ian Osterloh suggested that the drug had little effect on angina, but that it could induce marked penile erections. Pfizer therefore decided to market it for erectile dysfunction, rather than for angina. The drug was patented in 1996, approved for use in erectile dysfunction by the Food and Drug Administration on March 27, 1998, becoming the first pill approved to treat erectile dysfunction in the United States, and offered for sale in the United States later that year. It soon became a great success: annual sales of Viagra in the period 1999–2001 exceeded $1 billion. Finding a Lead (cont.) O N N N NH O O S O N N viagra (Sildenafil) Structure-Activity-Relationships (SAR’s) • Once a lead has been discovered, it is important to understand precisely which structural features are responsible for its biological activity (i.e. to identify the “pharmacophore”) The pharmacophore is the precise section of the molecule that is responsible for biological activity • This may enable one to prepare a more active molecule • This may allow the elimination of “excessive” functionality, thus reducing the toxicity and cost of production of the active material • This can be done through synthetic modifications • Example: R-OH can be converted to R-OCH3 to see if O-H is involved in an important interaction • Example: R-NH2 can be converted to R-NH-COR’ to see if interaction with positive charge on protonated amine is an important interaction Link Next step: Improve Pharmacokinetic Properties • Improve pharmacokinetic properties. pharmacokinetic = The study of absorption, distribution, metabolism and excretion of a drug (ADME). • Video • exercise=MedicationDistribution&title=Medica tion%20Absorption,%20Distribution,%20Meta bolism%20and%20Excretion%20Animation& publication_ID=2450 Metabolism of Drugs • The body regards drugs as foreign substances, not produced naturally. • Sometimes such substances are referred to as “xenobiotics” •Body has “goal” of removing such xenobiotics from system by excretion in the urine •The kidney is set up to allow polar substances to escape in the urine, so the body tries to chemically transform the drugs into more polar structures. Metabolism of Drugs (cont.) • Phase 1 Metabolism involves the conversion of nonpolar bonds (eg C-H bonds) to more polar bonds (eg C-OH bonds). • A key enzyme is the cytochrome P450 system, which catalyzes this reaction: RH + O2 + 2H+ + 2e– → ROH + H2O Mechanism of Cytochrome P450 Phase I metabolism may either detoxify or toxify. • Phase I reactions produce a more polar molecule that is easier to eliminate. • Phase I reactions can sometimes result in a substance more toxic than the originally ingested substance. • An example is the Phase I metabolism of acetonitrile The Liver • Oral administration frequently brings the drugs (via the portal system) to the liver Metabolism of Drugs (cont.) • Phase II metabolism links the drug to still more polar molecules to render them even more easy to excrete UDP Glucuronic Acid Glucuronic Acid HO O HO O O P O P O O O NH O glucuronosyltransferase enzyme N HO OH OH O Drug O HO R O HO HO O O HO OH OH OH More easily excreted than ROH itself R OH Drug Metabolism of Drugs (cont.) • Another Phase II reaction is sulfation (shown below) NH2 N O O R OH S O- N O O P O N O O- SO3- N R Drug O O P OH O- O- 3'-Phosphoadenosine-5'-phosphosulfate O Sulfated Drug (more easily excreted) Phase II Metabolism • Phase II reactions most commonly detoxify • Phase II reactions usually occur at polar sites, like COOH, OH, etc. Manufacture of Drugs • • • • Pharmaceutical companies must make a profit to continue to exist Therefore, drugs must be sold at a profit One must have readily available, inexpensive starting materials One must have an efficient synthetic route to the compound – As few steps as possible – Inexpensive reagents • The route must be suitable to the “scale up” needed for the production of at least tens of kilograms of final product • This may limit the structural complexity and/or ultimate size (i.e. mw) of the final product • In some cases, it may be useful to design microbial processes which produce highly functional, advanced intermediates. This type of process usually is more efficient than trying to prepare the same intermediate using synthetic methodology. Toxicity • Toxicity standards are continually becoming tougher • Must use in vivo (i.e. animal) testing to screen for toxicity – Each animal is slightly different, with different metabolic systems, etc. – Thus a drug may be toxic to one species and not to another Example: Thalidomide Thalidomide was developed by German pharmaceutical company Grünenthal. It was sold from 1957 to 1961 in almost 50 countries under at least 40 names. Thalidomide was chiefly sold and prescribed during the late 1950s and early 1960s to pregnant women, as an antiemetic to combat morning sickness and as an aid to help them sleep. Before its release, inadequate tests were performed to assess the drug's safety, with catastrophic results for the children of women who had taken thalidomide during their pregnancies. Antiemetic = a medication that helps prevent and control nausea and vomiting Birth defects caused by use of thalidomide Example: Thalidomide From 1956 to 1962, approximately 10,000 children were born with severe malformities, including phocomelia, because their mothers had taken thalidomide during pregnancy. In 1962, in reaction to the tragedy, the United States Congress enacted laws requiring tests for safety during pregnancy before a drug can receive approval for sale in the U.S. O N O NH O O Thalidomide Phocomelia presents at birth very short or absent long bones and flipper-like appearance of hands and sometimes feet. Example: Thalidomide •Researchers, however, continued to work with the drug. Soon after its banishment, an Israeli doctor discovered antiinflammatory effects of thalidomide and began to look for uses of the medication despite its teratogenic effects. •He found that patients with erythema nodosum leprosum, a painful skin condition associated with leprosy, experienced relief of their pain by taking thalidomide. Teratogenic = Causing malformations in a fetus Thalidomide Further work conducted in 1991 by Dr. Gilla Kaplan at Rockefeller University in New York City showed that thalidomide worked in leprosy by inhibiting tumor necrosis factor alpha. Kaplan partnered with Celgene Corporation to further develop the potential for thalidomide. Subsequent research has shown that it is effective in multiple myeloma, and it is now approved by the FDA for use in this malignancy. There are studies underway to determine the drug's effects on arachnoiditis, Crohn's disease, and several types of cancers. Clinical Trials • Phase I: Drug is tested on healthy volunteers to determine toxicity relative to dose and to screen for unexpected side effects Clinical Trials • Phase II: Drug is tested on small group of patients to see if drug has any beneficial effect and to determine the dose level needed for this effect. Clinical Trials • Phase III: Drug is tested on much larger group of patients and compared with existing treatments and with a placebo Clinical Trials • Phase IV: Drug is placed on the market and patients are monitored for side effects Assigned Reading • Haffner Marlene E; Whitley Janet; Moses Marie Two decades of orphan product development. Nature reviews. Drug discovery (2002), 1(10), 821-5. Link • Franks Michael E; Macpherson Gordon R; Figg William D Thalidomide. Lancet (2004), 363(9423), 1802-11. Link • Abou-Gharbia, Magid. Discovery of innovative small molecule therapeutics. Journal of Medicinal Chemistry (2009), 52(1), 2-9. Link Optional Additional Reading • Bartlett J Blake; Dredge Keith; Dalgleish Angus G The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nature reviews. Cancer (2004), 4(4), 314-22. Link • Cragg, G. M.; Newman, D. J. Nature: a vital source of leads for anticancer drug development. Phytochemistry Reviews (2009), 8(2), 313-331. Link Homework Questions • For each of the top twelve pharmaceutical companies (see presentation 1), list the top selling drug of that company, its brand and generic name, and draw its structure. • What is an ‘orphan drug’. Why has the Orphan Drug Act been successful? • Thalidomide is actually a mixture of two compounds. Draw their structures and list the physiological effects of each. • What does ADMET stand for? • List several possible reasons for poor efficacy of drug candidates in in vivo models.