* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download interaction of drugs with biological matrices
Survey
Document related concepts
Transcript
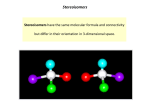
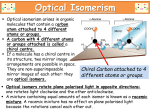
Stereokimia Kenapa perlunya mempelajari stereokimia? Pharmacological activity of compounds(drugs) depend mainly on their interaction with biological matrices (drug targets), such as proteins (receptors, enzymes), nucleic acids (DNA and RNA) and biomembranes (phospholipids and glycolipids). All these matrices have complex three-dimensional structures which are capable to recognize (bind) specifically the ligand (drug) molecule in only one of the many possible arrangements in the three-dimensional space. It is the three-dimensional structure of the drug target that determines which of the potential drug candidate molecules is bound within its cavity and with what affinity. This section concerns factors which control three-dimensional shape of organic molecules (drugs) viewed from the perspective of their interaction with potential biological targets. Stereokimia Why is drug chirality an important knowledge for future pharmacists? The current trend in drug markets is a rapid increase of the sales of chiral drugs at the expense of the achiral ones. By the year 2000 chiral drugs, whether enantiomerically pure or sold as a racemic mixture, will dominate drug markets. It is therefore important to understand how drug chirality affects its interaction with drug targets and to be able to use proper nomenclature in describing the drugs themselves and the nature of forces responsible for those interactions. EXAMPLES OF DRUGS SOLD AS SINGLE ENANTIOMERS • Levomoprolol/Levotensin • Levodropropizine/Levotuss • Levofloxacin/Cravit • Barnidipine/Hypoca • Dexfenfluramine/Isomeride • Ibuprofen/Serectil FACTORS THAT MAKE UP 3D-STRUCTURES OF RECEPTORS AND DRUGS The importance of most factors affecting the 3D-shape of the drug and receptor molecules is illustrated on the example of the oligopeptide chain above. Such an oligopeptide is a linear molecule which is built by atoms separated from each other by a certain distance (bond length), endowed with a certain charge (due to bond polarity) or hydrophobicity (a property of repelling water), related to more distant sites in the molecules by a rotation angle about the single bond, and featuring more rigid fragments such as bonds with partial double bond characters. In addition, the orientation of the R group (amino acid side chain) is very important (chirality of the amino acid), as it determines the shape of the cavities lined with the oligopeptide chains. These factors are itemized below: GLOSSARY OF TERMS RELATED TO 3D-STRUCTURE OF MOLECULES STRUCTURE is the complete arrangement of all the atoms of the molecule in space as determined by such methods as X-ray diffraction (as defined by Cartesian coordinates of all atoms). This terms is the broadest one and includes constitution (nature of atoms, their number, the type of bonds and manner in which they are linked together(connectivity)), conformation and configuration. CONFORMATION is the spatial arrangement of atoms in the molecule of the given constitution and configuration. Conformation can be changed without changes in constitution and configuration by a rapid(?) rotation about single bonds and pyramidal inversion(?) at some centers. CONFIGURATION is the spatial arrangement of atoms that distinguishes molecules of the same constitution (isomers), other than distinction due to differences in the conformation. SYMMETRY is a regular occurrence of certain patterns within an object or structure (at macro- or microscopic level). These patterns are generated by the presence of symmetry elements such as center of symmetry; symmetry axes; symmetry planes. GLOSSARY OF TERMS RELATED TO 3D-STRUCTURE OF MOLECULES CHIRALITY is a property of an object which is non-superimposable with its mirror image. Most objects in the environment are chiral. In chemistry this term applies to molecules, specific conformations of molecules, as well as to macros copic objects such as crystals. Chirality is removed if and object molecule acquires a plane of symmetry, or a center of symmetry. The molecule can remain chiral with a limited combination of symmetry axes. ENANTIOMERS are molecules related to each other as a real object to its mirror image. Enantiomers are therefore related to each other through the reflection by the mirror plane, and are not superposable. Not all object/mirror-image pairs constitute enantiomers, but only those which are not superimposable after any rotation/translation of the whole object, or its mirror image. Enantiomeric relation does not bear the aspect of energy; the conformational isomers existing in the fast interconversion are still considered enantiomers. For the purpose of the determination whether two conformations are enantiomeric, they are considered to be rigid. The existence of enantiomers is usually (but not always) associated with at least one chiral center. Enantiomers have exactly the same energies, and therefore are not differentiated by physical measurements other than optical rotation (rotation of the plane of polarized light). GLOSSARY OF TERMS RELATED TO 3D-STRUCTURE OF MOLECULES DIASTEREOMERS are any molecules which have an identical constitution, but are not related through the mirror reflection operation. Diastereomers could be compounds with two or more chiral centers, in which not all chiral centers have opposite configuration to a corresponding chiral centers in another molecule (the whole molecule would be the mirror image of the other and thus an enantiomer). Diastereomers do not have to possess chiral center(s), they only need to differ by a spatial difference not related to mirror reflection. Thus, diastereomers could be nonchiral cis/trans -isomers of cyclic or olefinic (alkene) compounds. Diastereomers and enantiomers are frequently jointly referred to as stereoisomers. STEREOISOMERS: a combined term including enantiomers and diastereomers SYMMETRY ELEMENTS 1. Center of Symmetry The center of symmetry i is a point in space such that if a line is drawn from any part (atom) of the molecule to that point and extended an equal distance beyond it, an analogous part (atom) will be encountered. This symmetry element is sometimes also called "the point of inversion" SYMMETRY ELEMENTS 2. Plane of Symmetry Planes, centers and alternating axes correspond to "symmetry operations of the second kind" or "improper operations" since they bring into coincidence the material point of an object with its mirror reflection. A plane of symmetry is a reflection plane which brings into coincidence one point of the molecule with another one through the mirror reflection. SYMMETRY ELEMENTS 2. Plane of Symmetry Planes, centers and alternating axes correspond to "symmetry operations of the second kind" or "improper operations" since they bring into coincidence the material point of an object with its mirror reflection. A plane of symmetry is a reflection plane which brings into coincidence one point of the molecule with another one through the mirror reflection. SYMMETRY ELEMENTS 3. Axis of Symmetry Symmetry axis Cn, also called n-fold axis, is an axis which rotates the object (molecule) around by 360ø/n, such that the new position of an object is superimposable with the original one. GRAPHICAL REPRESENTATION OF NONPLANAR MOLECULES Fisher projection: The tetrahedral atom is viewed perpendicularly to an edge formed by connecting two of its ligands. The convention is that the two vertical bonds in the projection are pointing behind the plane of projection (plane of paper sheet), and the two horizontal Wedge projection It is obtained by viewing the tetrahedral center perpendicularly to the plane formed by three atoms. One of the remaining atoms is oriented behind the plane of projection (dashed bond), one towards the viewer (boldface bond). Note that, in contrast to Fisher projection, the rotation of the wedge projection about axes perpendicular or coplanar with the plane of projection does not change anything. This projection is therefore by far less ambiguous than Fisher projection. In the case of large linear molecule the molecule backbone has to be drawn in the fully extended conformation. Newman projection: The molecule with two tetrahedral centers is viewed along the C-C axis. The atom in front is represented as a three-way branch, the atom in the back as a circle with three outgoing bonds. This projection is most useful inconsideration of steric relation between ligands linked to adjacent tetrahedral centers and is most popular. . Sawhorse projection: The C-C bond is viewed at an angle. The atom shown on the left of the projection is also the one in the front. This projection is difficult to use with acyclic molecules but is most popular for representation of cyclic molecules e.g. saturated six-membered rings . . Haworth projection: . INTERACTION OF DRUGS WITH BIOLOGICAL MATRICES Chirality (enantiomerism) It is very important from the point of view of drug development and its mechanism of action. Since most of the natural (biological) environment consists of enantiomeric molecules (aminoacids, nucleosides, carbohydrates and phospholipids are chiral molecules) it makes sense that drugs developed are also chiral. Frequently only one stereoisomer is active, and sometimes the other one is toxic (the current policies of FDA in drug approval is that the inactive enantiomer in the racemic drug has to be shown to be devoid of any toxicity or undesired side-effects). A drug upon administration undergoes a series of steps before exerting its activity. At each step the structure of the drug and hence its chirality influences the further metabolism INTERACTION OF DRUGS WITH BIOLOGICAL MATRICES . INTERACTION OF DRUGS WITH BIOLOGICAL MATRICES The reason for chiral recognition by drug receptors is a three-point interaction of the agonist or substrate with the receptor or enzyme active site, respectively. . INTERACTION OF DRUGS WITH BIOLOGICAL MATRICES Example: Only the (-) enantiomer of epinephrine has the OH group in the binding site, and therefore has a much more potent pressor activity . INTERACTION OF DRUGS WITH BIOLOGICAL MATRICES The D(-) lactoyl choline is hydrolyzed much more slowly than the L(+)-isomer due to favorable binding of the OH group in the latter case. . INTERACTION OF DRUGS WITH BIOLOGICAL MATRICES Likewise, cis/trans isomers of cyclic compounds, or Z/E isomers of alkenes are also expected to have different binding potency and therefore also different biological activity. . RULES FOR SPECIFICATION OF CHIRALITY 1. Chiroptical properties Any material which rotates the plane of the polarized light is termed "optically active." Compounds featuring chiral centers are optically active unless they possess symmetry plane or a symmetry center (see above). An isomer of optically active compound can rotate the plane of polarized light to the left (levorotatory), in which case it will be designated (l, or -), or to the right (dextrorotatory) in which case it will be termed (d, or +). There are following properties associated with enantiomerism: 1. Enantiomeric molecules interact in a different manner with another enantiomeric molecules (such as biological receptors, but also with simple chiral organic molecules); it regards both weak interaction such as forming weak complexes, as well as chemical reactions (bond breaking or forming). 2. Enantiomers can not be distinguished by their interactions with achiral molecules, nor by their physical properties measured by techniques other than those using in-plane-, or circularly-polarized light. 1. Chiroptical properties Several rules for specifying chirality have been adopted from the time of van't Hoff. The L/D systems relies on the chemical correlation of the configuration of the chiral center to D-glyceraldehyde. The compounds which can be correlated without inverting the chiral center are named D (capital D), those correlated to its enantiomer are designated as L (capital L). IMPORTANT NOTE: Although D-glyceraldehyde is dextrorotatory (rotates the plane of polarized light to the right), the compounds correlated to D-glyceraldehyde do not have to be dextrorotatory, i.e. could rotate light to the left. Therefore, D-prefix is not correlated with the (+) or (-) specific rotation, and the D-compound can be l, (or -), and vice versa L-compound can be d (or +). This nomenclature system is slowly being abandoned in favor of the Cahn-Ingold-Prelog (CIP) nomenclature, with the exception where the DL-nomenclature has been used traditionally, and is more useful (D-carbohydrates or L-aminoacids). 1. Chiroptical properties EXAMPLE: An antiinflammatory agent such as Ibuprofen has two configurations(R and S). However, only S-configuration has pharmacologic properties. The demonstration(video) of superposition between the S and R configurations indicates that they are enantiomers. They are nonsuperposable mirror reflection of each other 2. Cahn-Ingold-Prelog Rules For the tetrahedral chiral center C with four inequivalent substituents the rule is adopted for designation of chirality. • First ligands are ordered by the ligand precedence rules as 1,2,3 & 4. • The central atom and three ligands are viewed from the direction of C 4 vector where 4 is a ligand of lowest precedence. • If the ligands 1-3 are ordered such that the movement from the ligand of the highest precedence (1) to the third (3) passing the second (2) in between is in the clockwise direction (sequence 1 2 3) the configuration is designated as R (rectus, written in italic, capital). On the other hand if the same requires movement in the anti clockwise direction the configuration is designated as S (sinister). The configurational designation is preceded by the number specifying the location of the chiral center, i.e. 2R with one chiral center at the 2-position and R configuration, or 2R, 3S, 4R with multiple chiral centers. 3. Ligand precedence rules Ligands of the higher atomic number precede those with lower ones, e.g. Br precedes Cl (Br>Cl). 1. For ligands with the same type of atoms linked to the center C, the precedence is determined based on the atomic numbers of ligands in the next sphere, e.g. ligand with C-O sequence precedes C-C. If no difference is detected, the determination is based on the distinction in the next spheres, and search is continued until the difference is detected. 2. The coordination number of non-hydrogen atoms is assumed to be 4, i.e. atoms bonded with multiple bonds are considered to be bonded to multiple atoms, e.g. carbonyl carbon is treated as if it was bonded to two oxygen atoms, and carboxyl carbon as if it was bonded to three oxygens (these are then called phantom atoms). Ligand duplication is also necessary in the cases of cyclic systems 3. Ligands of the same atomic number, but a higher atomic mass precede those with a lower atomic mass, e.g. D precedes H (D>H). This criterion applies only after the previous ones were exhausted. 4. For compounds where only configurational (not constitutional) differences between ligands are detected, the following rules apply: • The olefinic ligand that has the chiral center and another ligand on the same side of the double bond (cis) precedes the one with the trans-configuration. • Ligands with R,R or S,S precede R,S or S,R. • R precedes S 3. Ligand precedence rules . 4. Helical Chirality Certain natural, as well as unnatural linear polymers assume helical conformation: e.g. right-handed B-DNA, left-handed Z-DNA, protein alpha-helices. The hydrated lipids can form chiral mesophases, whereby chirality is due to small rotation of each layer versus the adjacent layers in the multilayer stack. These macromolecules or aggregates are said to have helical chirality. The chirality of such compounds is determined by determining the screw sense of the helix. If the screw is right handed the chirality is P(plus), if it is left handed the chirality is M(minus). Conformations of simple chain compounds can also be treated as if they had helical chirality 5. Z/E Geometry of Double Bonds The same Prelog's precedence rules, as discussed earlier, apply to geometrical isomers of olefinic compounds and alicyclic compounds. Precedence of ligands at both nodes of the double bonds is determined pairwise. If both higher precedence ligands are on the same side of the double bond the configuration is Z, if on the opposite sides the configuration is E. 6. cis/trans Geometry of Alicyclic Compounds The cyclic systems use the traditional cis/trans nomenclature. When specifying geometry (cis/trans) the precedence of substituents is also determined based on the precedence rules set forth earlier. The situation is simple in the case of disubstituted systems, however, in the case of multiple substitution the geometry of the ring system has to be specified with respect to a selected reference indicated by the the symbol "r" (italic). Example: 7. Relative Configurations in Compounds with Multiple Chiral Centers. The most unambiguous notation employs Prelog's descriptors R,S. For example for D-glucose (below) the correct specification of chirality is 2R, 3S, 4R, 5S. Note that only R,S descriptors are italicized and the chirality is specified with the numerical prefix denoting this position. The configuration of racemic compounds is specified by using RS notation for each chiral center. Note that the relative configurations have to be preserved in order to correspond to a given diastereomers Thus racemic glucose would be described as 2RS, 3SR, 4RS, 5SR. (in contrast, 2SR, 3SR, 4RS, 5SR would correspond to racemic mannose). 7. Relative Configurations in Compounds with Multiple Chiral Centers. The use of CIP nomenclature requires assignment of R,S descriptors for every center. The quicker way (older and a more ambiguous one) is by using threo/erythro nomenclature. This notation is based on the four-carbon sugars threose and erythrose. It requires vertical projection of the sugar main chain; threo-compounds are defined as those that have two ligands of higher precedence on each carbon atom on the opposite sides of the chain, erythro on the same side. The ambiguity arises from the question what should be used as the main chain 8. Mezo Compounds and Pseudoasymmetry In compounds in which two or more chiral ligands of the central atom are constitutionally identical but have the opposite configuration the central atom is formally chiral because it has four different ligands (even though the difference is only in ligand configuration). However, since such a compound also has a plane of symmetry it is, in fact, achiral as a whole. The central atom is termed a pseudoasymmetric center. The configuration of such atom is determined according to normal precedence rules assuming that R precedes S. In contrast, in molecules in which the two ligands of the central atom havethe same configuration the central atom is achiral, but the molecule CONFORMATION DEFINITION Conformation is a spatial arrangement of a molecule of a given constitution and configuration. In the case of a four atom molecule linked in a chain manner, rotation of atom A or D about the inner B-C bond by an angle leads to a different mutual relation of atom A and D and results in population of a set of different rotational isomers or "conformations". The single parameter differentiating such conformers is an angle between two planes that contain atoms ABC and BCD in themselves. This dihedral angle is called a "torsion" angle and is most frequently used for specification of the type of conformations. The conformation of a molecule containing two tetrahedral atoms linked together can be represented as a "sawhorse" or as a Newman projections. In the Newman projection the molecule is viewed along the axis of a rotatable bond. Isomers . Isomers 1. STRUCTURAL ISOMERISM a. Chain isomerism b. Position isomerism c. Functional group isomerism 2. STEREOISOMERS a. Enantiomer - atropisomer b. Diastereomers - Two or more sterocentre - Geometric (cis / trans) isomerism Geometric (cis / trans) isomerism How geometric isomers arise To get geometric isomers you must have: • restricted rotation (involving a carbon-carbon double bond) • two different groups on the left-hand end of the bond and two different groups on the right-hand end. It doesn't matter whether the left-hand groups are the same as the right-hand ones or not. Geometric (cis / trans) isomerism The effect of geometric isomerism on physical properties • The table shows the melting point and boiling point of the cis and trans isomers of 1,2-dichloroethene. Isomers Cis Trans • • Melting point (0C) -80 -50 Boiling point(0C) 60 48 The trans isomer has the higher melting point;the cis isomer has the higher boiling point. Both of the isomers have exactly the same atoms joined up in exactly the same order. That means that the van der Waals dispersion forces between the molecules will be identical in both cases.The difference between the two is that the cis isomer is a polar molecule whereas the trans isomer is non-polar. Geometric (cis / trans) isomerism • • • Both of the isomers have exactly the same atoms joined up in exactly the same order. That means that the van der Waals dispersion forces between the molecules will be identical in both cases.The difference between the two is that the cis isomer is a polar molecule whereas the trans isomer is nonpolar. Both molecules contain polar chlorine-carbon bonds, but in the cis isomer they are both on the same side of the molecule. That means that one side of the molecule will have a slight negative charge while the other is slightly positive. The molecule is therefore polar. Because of this, there will be dipole-dipole interactions as well as dispersion forces - needing extra energy to break. That will raise the boiling point Geometric (cis / trans) isomerism Enantiomers: A mixture of these cannot be separated by normal GC or HPLC techniques Diastereomers: Different physical/chemical properties in chiral/achiral environments. A mixture of these can be separated by normal GC or HPLC techniques Geometric (cis / trans) isomerism Stereogenic center (stereocenter) – a point in a molecule bearing groups such that an interchange of any two groups will produce a stereoisomer. Number of possible stereoisomers = 2n , n = # of stereocenters. Relative vs. Absolute Configurations • Relative configuration - 3-D structure is not known but it is known that one structure is the mirror image of the other. • (+) or d - dextrorotatory - cpd that rotates light to the right. • (-) or l - levorotatory - cpd that rotates light to the left. • (±) - racemic mixture - 1:1 mixture of (+) and (-) cpds, zero rotation. • Enantiomeric excess: • Absolute configuration - 3-D structure is known. R, S Nomenclature (-)-alanine 1. a. b. 2. 3. Prioritize - assign a priority to the groups around the stereocenter increasing atomic mass of the atom attached to stereocenter in case of a tie move to the next atom Place - place the lowest priority group in the back. Connect a b c Separation of Enantiomers A) Resolution B) Pasteur's Method