* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Introduction to Bioequivalence Studies
Hormonal contraception wikipedia , lookup
Plateau principle wikipedia , lookup
Drug interaction wikipedia , lookup
Drug design wikipedia , lookup
Zoopharmacognosy wikipedia , lookup
Compounding wikipedia , lookup
Pharmaceutical marketing wikipedia , lookup
Drug discovery wikipedia , lookup
Environmental persistent pharmaceutical pollutant wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacognosy wikipedia , lookup
Theralizumab wikipedia , lookup
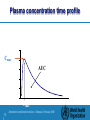
Training workshop on regulatory requirements for registration of Artemisinin based combined medicines and assessment of data which are submitted to regulatory authorities Why are bioavailability / bioequivalence studies necessary? An Introduction to Bioequivalence Studies Presented by: Hans Kemmler, Consultant to WHO Accra, 5.Nov. 2008 1| Artemisinin combined medicines, Kampala, February 2009 Background: First Product to Market Innovator’s Product Quality Safety and efficacy – Based on extensive clinical trials – Expensive – Time consuming 2| Artemisinin combined medicines, Kampala, February 2009 Background: Other products with same medicinal ingredient Subsequent-entry products Generic products Multisource products How do these products gain marketing authorization? 3| Artemisinin combined medicines, Kampala, February 2009 Pharmaceutical equivalence Same amount of the same active pharmaceutical ingredient – Salts, esters Same dosage form – Comparable dosage forms – e.g., tablet vs. capsule Same route of administration Is pharmaceutical equivalence enough? 4| Artemisinin combined medicines, Kampala, February 2009 Sometimes pharmaceutical equivalence is enough Aqueous solutions – – – – – – Intravenous solutions Intramuscular, subcutaneous Oral solutions Otic or ophthalmic solutions Topical preparations Solutions for nasal administration Powders for reconstitution as solution Gases 5| Artemisinin combined medicines, Kampala, February 2009 Sometimes it is not enough Pharmaceutical equivalence by itself does not necessarily imply therapeutic equivalence Therapeutic equivalence: – Pharmaceutically equivalent – Same safety and efficacy profiles after administration of same dose 6| Artemisinin combined medicines, Kampala, February 2009 Pharmaceutical Equivalents Reference Test Possible Differences Drug particle size Excipients Manufacturing Equipment or Process Site of manufacture Could lead to differences in product performance in vivo 7| Artemisinin combined medicines, Kampala, February 2009 Additional data is required Oral immediate release products with systemic action – Generally required for solid oral dosage forms • • • • 8| Critical use Narrow therapeutic range Bioavailability problems associated with the active ingredient Problematic polymorphism, excipient interaction, or sensitivity to manufacturing processes Artemisinin combined medicines, Kampala, February 2009 Additional data is required Oral modified release products with systemic action Fixed dose combination products with systemic action – When at least one component requires study Non-oral / non-parental products with systemic action Non-solution products with non-systemic action 9| Artemisinin combined medicines, Kampala, February 2009 Marketing authorization of multisource products Extensive clinical trials to demonstrate safety and efficacy – Interchangeability? Demonstration of equivalence to reference (comparator) product – Interchangeability – Therapeutic equivalence 10 Artemisinin combined medicines, Kampala, February 2009 Marketing authorization through equivalence Suitable methods for assessing equivalence: – – – – 11 Comparative pharmacokinetic studies Comparative pharmacodynamic studies Comparative clinical trials Comparative in vitro tests Artemisinin combined medicines, Kampala, February 2009 Comparative Pharmacokinetic Studies In vivo measurement of active ingredient “Some” relationship between concentration and safety/efficacy Product performance is the key Comparative bioavailability 12 Artemisinin combined medicines, Kampala, February 2009 Bioavailability The rate and extent to which a substance or its active moiety is delivered from a pharmaceutical form and becomes available in the general circulation.” Reference: intravenous administration = 100% bioavailability 13 Artemisinin combined medicines, Kampala, February 2009 Important Pharmacokinetic Parameters AUC: area under the concentration-time curve measure of the extent of bioavailability Cmax: the observed maximum concentration of drug measure of both the rate of absorption and the extent of bioavailability tmax: the time after administration of drug at which Cmax is observed measure of the rate of absorption 14 Artemisinin combined medicines, Kampala, February 2009 Plasma concentration time profile concentration Cmax AUC Tmax 15 Artemisinin combined medicines, Kampala, February 2009 time Bioequivalence Two products are bioequivalent if they are pharmaceutically equivalent bioavailabilities (both rate and extent) after administration in the same molar dose are similar to such a degree that their effects can be expected to be essentially the same 16 Artemisinin combined medicines, Kampala, February 2009 Bioavailability Absolute bioavailability (F): AUCextravascular Doseint ravenous F AUCint ravenous Doseextravascular Relative bioavailability (Frel) AUCextravascular1 Doseextravascular 2 Frel AUCextravascular 2 Doseextravascular1 17 Artemisinin combined medicines, Kampala, February 2009 Bioavailability: Same Dose Absolute bioavailability (F): AUCextravascular Doseint ravenous F AUCint ravenous Doseextravascular Relative bioavailability (Frel) AUCextravascular1 Doseextravascular 2 Frel AUCextravascular 2 Doseextravascular1 18 Artemisinin combined medicines, Kampala, February 2009 Therapeutic Equivalence Therapeutic equivalence: – Pharmaceutically equivalent – Same safety and efficacy profiles after administration of same dose: bioequivalent Interchangeability 19 Artemisinin combined medicines, Kampala, February 2009 Comparative Pharmacodynamic Studies Not recommended when: – active ingredient is absorbed into the systemic circulation – pharmacokinetic study can be conducted Local action / no systemic absorption 20 Artemisinin combined medicines, Kampala, February 2009 Comparative Clinical Studies Pharmacokinetic profile not possible Lack of suitable pharmacodynamic endpoint Typically insensitive 21 Artemisinin combined medicines, Kampala, February 2009 Comparative in vitro Studies May be suitable in lieu of in vivo studies under certain circumstances Requirements for waiver to be discussed 22 Artemisinin combined medicines, Kampala, February 2009 When are bioequivalence studies employed? Multisource product vs. Innovative product Pre-approval changes – Bridging studies Post-approval changes Additional strengths of existing product 23 Artemisinin combined medicines, Kampala, February 2009 Bioequivalence Studies: Basic Design Considerations Minimize variability not attributable to formulations Minimize bias REMEMBER: goal is to compare performance of the two products 24 Artemisinin combined medicines, Kampala, February 2009 “Gold Standard” Study Design Single-dose, two-period, crossover Healthy volunteers Subjects receive each formulation once Adequate washout 25 Artemisinin combined medicines, Kampala, February 2009 Multiple-dose Studies More relevant clinically? Less sensitive to formulation differences 26 Artemisinin combined medicines, Kampala, February 2009 Multiple-dose Studies may be employed when: Drug is too potent/toxic for administration in healthy volunteers – Patients / no interruption of therapy Extended/modified release products – Accumulation using recommended dosing interval – In addition to single-dose studies 27 Artemisinin combined medicines, Kampala, February 2009 Multiple-dose Studies may be employed when: Non-linear pharmacokinetics at steady-state (e.g., saturable metabolism) Assay not sufficiently sensitive for single-dose study 28 Artemisinin combined medicines, Kampala, February 2009 Crossover vs. Parallel Designs Crossover design preferred – Intra-subject comparison – Lower variability – Generally fewer subjects required Parallel design may be useful – Drug with very long half-life – Crossover design not practical 29 Artemisinin combined medicines, Kampala, February 2009 Parallel Design Considerations Ensure adequate number of subjects Adequate sample collection – Completion of Gastrointestinal transit / absorption process – 72 hours normally sufficient 30 Artemisinin combined medicines, Kampala, February 2009 Fasted vs. Fed Designs Fasted study design preferred – Minimize variability not attributable to formulation – Better able to detect formulation differences 31 Artemisinin combined medicines, Kampala, February 2009 Fed Study Designs may be employed when: Significant gastrointestinal (GI) disturbance caused by fasted administration Product labeling restricts administration to fed state 32 Artemisinin combined medicines, Kampala, February 2009 Fed Study Design Considerations Fed conditions depend on local diet and customs Dependent on reason for fed design – Avoiding GI disturbance • Minimal meal to minimize impact – Required due to drug substance / dosage form • Modified-release products • Complicated pharmacokinetics • Known effect of food on drug substance 33 Artemisinin combined medicines, Kampala, February 2009 Fed Study Design Considerations cont. Fed conditions designed to promote maximal perturbation – High fat – High Calorie – Warm 34 Artemisinin combined medicines, Kampala, February 2009 Replicate vs. non-replicate designs Standard approach – Non-replicated – Single administration of each product – Average bioequivalence 35 Artemisinin combined medicines, Kampala, February 2009 Replicate Designs Typically four-period design – Each product administered twice Intra-subject variability Subject X formulation interaction Different approaches possible – Average bioequivalence – Individual bioequivalence 36 Artemisinin combined medicines, Kampala, February 2009 Replicate Designs Advantages – More information available – Different approaches to assessment possible Disadvantages – Bigger commitment for volunteers – More administrations to healthy volunteers – More expensive to conduct 37 Artemisinin combined medicines, Kampala, February 2009 Discussion Questions Comments Opinions 38 Artemisinin combined medicines, Kampala, February 2009









































![My_Body[1] - Junior2TopicWiki](http://s1.studyres.com/store/data/008060165_1-be31cd2568d5e2c9fee6ce67732b07b4-150x150.png)





