* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download No Slide Title
Polysubstance dependence wikipedia , lookup
Drug design wikipedia , lookup
Psychopharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug discovery wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Neuropharmacology wikipedia , lookup
Theralizumab wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacokinetics wikipedia , lookup
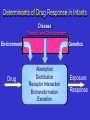
PHARMACOGENOMICS John N. van den Anker, MD, PhD, FCP, FAAP Children’s National Medical Center, Washington, DC, USA & Intensive Care, Erasmus MC-Sophia Children’s Hospital, Rotterdam, the Netherlands Disclosure presentation John N. van den Anker I do not have anything to disclose related to the content of my presentation Individual variability in drug response can have serious consequences Stevens-Johnson Syndrome (SJS) Adverse Drug Reaction The Ideal Medication Effectively treats or prevents disease Has no adverse effects Paradox of Modern Drug Development 1. Clinical trials provide evidence of efficacy and safety at usual doses in populations + = Efficacious & Safe 2. Physicians treat individual patients who can vary widely in their response to drug therapy No Response + = Efficacious & Safe Adverse Drug Reaction Adverse Drug Reactions • 4-6th leading cause of death in the USA1 • Health care costs: $137-177 billion annually (USA)2-3 • Cause 7% of all hospital admissions4 • Cause serious reactions in over 2,000,000 hospitalized patients (6.7%) each year in the USA1 • Cause fatal reactions in over 100,000 hospitalized patients each year in the USA1 • 50% of newly approved therapeutic health products have serious ADRs, discovered only after the product is on the market (Health Canada, 2007) • 95% of all ADRs are unreported 1. Lazarou et al, JAMA, 1998 2. Johnson et al, Arch Intern Med 1995 3. Ernst et al, J. Am. Pharm. Assoc. 2001 4. Pirmohamed et al, BMJ, 2004 5. MjoÈrndal et al, EACPT3, 1999 Factors Contributing to Variability in Drug Response Gender Weight Ethnicity Diet Compliance Genetic Factors 20-95% Concomitant Disease Concomitant Drugs Age Patient genotype is currently an unknown factor in the prescribing of medicines Determinants of Drug Response in Infants Disease Growth and Development Environment Drug Genetics Absorption Distribution Receptor Interaction Biotransformation Excretion Exposure Response The Challenge of Optimizing the Use of Medicines in Paediatric Patients: Determining the Source(s) of Variability…... Ontogeny Pharmacogenetics From DNA to mRNA to protein ATG ATC CCC TTT Met Ile Pro Phe 3 billion correct basepairs ….and 1 mutation • • atgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacga gttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccag gtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtac gtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcagga cgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtc caggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttca gtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcactacgtacatgtccaggtgca ggacgagttcagtacgtacatgtccaggtgcaggacgaattcagtacgtacatgtccaggtgcaggacgagttcagtacgtaca tgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagt tcagtacgtacatgtccaggtgcaggacgagttca gacgaattcagtacgtacatg atgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacga gttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccag gacgacttcagtacgtacatg gtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtac gtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcagga cgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtc caggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttca gtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgc aggacgagttcagtacgtacatgtccaggtgcaggacgacttcagtacgtacatgtccaggtgcaggacgagttcagtacgtac atgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacgagttcagtacgtacatgtccaggtgcaggacga gttcagtacgtacatgtccaggtgcaggacgagttca slow CYP2D6 intermediate rapid ultrarapid anti-depressants, anti-psychotics, anti-arrhythmics, beta-blockers, pain medications, anti-emetics, anti-cancer drugs CYP2C19 Poor metabolizer anti-convulsants, proton pump inhibitors, benzodiazepines, anti-malarials normal CYP2D6 Pharmacogenetics Drug Drug EM PM “Functional” overdose Stable metabolites, Excretion Stable metabolites, Excretion CYP2D6 Pharmacogenetics CYP2D6 activity displays bimodal distribution in Caucasian subjects 5-10% of Caucasian population deficient in CYP2D6 activity “Poor metabolizers” or “PMs” have two “inactive” forms (alleles) of the CYP2D6 gene PMs at increased risk for concentration- dependent side effects with “normal” drug doses Some drugs may not work (codeine; tramadol) CYP2D6 Pharmacogenetics: Caucasians Bertilsson et al. Clin. Pharmacol. Ther. 51:288-97, 1992 Number of Individuals 120 N = 1,011 80 40 12.6 0 0.01 Faster 0.1 1 CYP2D6 Activity 10 100 Slower CYP2D6 Activity: Chinese Bertilsson et al. Clin. Pharmacol. Ther. 51:288-97, 1992 Number of Individuals 120 N = 1,011 N = 695 80 40 12.6 0 0.01 Faster 0.1 1 CYP2D6 Activity 10 100 Slower Individuals Unravelling CYP2D6 Pharmacogenetics 40 30 20 EM Extensive Metabolizer Griese et al. Pharmacogenetics 1998, Raimundo et al. CPT 2004, Toscano et al. Pharmacogenetics 2006 UM ultrarapid metabolizer ~ 10-15 % IM Intermediate Metabolizer ~ 10-15 % PM Poor Metabolizer ~ 5-10 % Caucasians 10 0.1 1 10 100 MRS full-term healthy male infant day 7 pp: intermittent periods of difficulty in breastfeeding day 11: the baby had regained his birthweight day 12: grey skin, milk intake had fallen day 13: the baby was found dead autopsy: no abnormality blood concentration of morphine (metabolite of codeine): 70 ng/mL versus 0-2.2 ng/mL (typical) Pharmacogenetics of Codeine site of action codeine Cytochrome P450 2D6 morphine plasma morphine levels after 170 mg codeine p.o. morphine [pmol/ml] 60 50 Poor Metabolizer 40 Extensive Metabolizer 30 20 10 0 0 5 10 15 20 time [h] Eckhardt et al., Pain 1998 25 Explanation: medication mother due to episiotomy pain: codeine 60 mg plus paracetamol 1000 mg every 12 hrs for 2 weeks Morphine concentration in stored milk: 87 ng/mL mother: CYP2D6 genotype: CYP2D6*2x2 gene duplication = Ultra rapid metabolizer phenotype Prior to this publication! The American Academy of Pediatrics and “Drugs in Pregnancy in Lactation”, the major reference guide to fetal and neonatal risk, list codeine as compatible with breastfeeding – Briggs et al., 2005; Pediatrics, 2001 Estimated 1846 newborn infants are at risk for this codeine ADR annually in Canada (340,000 births, 73% breastfed, 52% mothers receive codeine post-childbirth,1.4% risk genotype) FDA drug label change and public health advisories Health Canada Public Advisory May 10, 2006 Aug. 21, 2008 Aug 17, 2007 August 20, 2009 2 year old boy Received tonsillectomy for sleep apnea Received standard codeine dose Died of respiratory depression High levels of morphine in blood Boy carried CYP2D6 gene duplication Kelly, Rieder, van den Anker et al. More codeine fatalities after tonsillectomy in North American children. Pediatrics 2012;129(5):1343-7 Transporters Receptors Phosphatases 2nd messengers Targets Protein kinases GI Lumen Blood Cell Opioids and pharmacogenomics Why personalizing opioid therapy? • Wide unpredictable interpatient variability • Narrow therapeutic indices Inadequate pain relief and side effects ~ 50% • Genetic factors: up to 60% (Angst 2012) Sadhasivam et al. (2012) Candidate genes 1. 410 pain genes 2. <10% translated to human pain 3. Opioid + genetic ≈ 2000 hits Division of genes • Pharmacokinetic: affect the availability at the site of action Phase I and II enzymes, transporters etc. • Pharmacodynamic: target and downstream signaling cascade Mu-opioid receptor, inwardly rectifying potassium channel etc • Pain sensitivity: susceptibility to pain Sodium channel, interleukines etc. PK RELATED GENES Metabolism fentanyl CYP3A4 Gene • Important drug metabolizing enzyme Highly expressed in liver and intestine broad substrate specificity (app. 50%) • Identified SNPs 22 alleles identified (CYPallele homepage) Rare or lack phenotypic effect • Caucasian *1G and *22 allele CYP3A4 SNPs CYP3A4*1G reduced activity higher plasma levels Less fentanyl required More side effects Studies Dong (2012),Yuan (2011) and Zhang (2010): Lower fentanyl requirement postoperative CYP3A4*22 Yuan (2011) correlation between plasma levels and requirement (r=-0.552, p<0.001) However, not associated with AEs and Pain score PD RELATED GENES OPRM1 and Fentanyl 118A>G • Higher fentanyl requirement (Zhang 2011) • Higher VAS pain score (Wu 2009) 118A>G relevant? Liao 2013: N=97, post-operative pain, fentanyl requirement+AEs CYP3A4*18 >> A118G 304A>G Lower fentanyl requirement (Landau 2009) Association with morphine requirement not found (Wong 2010) OPRM1 related genes Stat6 PAIN SENSITIVITY GENES Pain sensitivity genes Action potential • SCN9A Α-subunit Nav1.7 channel, nociceptive neurons R1150W increased sensitivity to pain (Reimann 2010) • KCNS1 Voltage gated K channel (Kv 9.1), sensory neurons 1465A>G increased sensitivity to pain (Costigan 2010) NICU study • n=132 • Mechanical ventilation (PNA<3 days) • 2 level III NICUs • Continous morphine vs placebo during max. 7 days • Loading dose 100 µg/kg 10 µg/kg/hr • Additional morphine (50 µg/kg 5-10 µg/kg/hr) Objective Determine if polymorphisms in PD related genes (OPRM1 118A>G, COMT Val158Met, ARRB2 8622C>T) are associated with additional morphine requirement (AMR) in newborns. Results NICU OPRM1 % AMR 118AA 31.0 118AG/118GG 34.3 COMT % AMR 158Val/Val 57.1 158Val/Met or 158Met/Met 26.9 ARRB2 % AMR 8622CC 11.1 8622CT/8622TT 34.3 OR* [95%CI] 4.93 [1.22-20] OR*[95%CI] 0.161 [0.04-0.650] OR* [95%CI] 5.52 [0.371-82.2] *corrected OR and 95%CI for postconceptional age, sex, allocation group, location centre . OPRM1 and COMT significant after Bonferroni correction Pharmacogenomics Avoid adverse drug reactions Maximize drug efficacy for individual patients Pharmacogenetic Profile: All Patients with Same Diagnosis High risk of ADR (50%): treat with alternative drug or dose Moderate risk of ADR (12.5%): treat with alternative drug or dose 10% risk of adverse reaction Low risk of ADR (0%): treat with conventional dose What do we need to do! All children are at risk for ADRs, but not all children are at equal risk. Find the kids at highest risk for serious ADRs due to genetic factors • Identify children with ADRs • Identify ‘matched’ children on same medications, without ADRs • Whenever possible, DNA samples are collected from biological parents of ADR patients • Look for genetic variation in key drug ADME enzymes • Develop new dosing guidelines • Bedside-benchtop-bedside science WE CAN’T TREAT CHILDREN LIKE ADULTS Increased Risk of Severe ADRs in Children >75% of approved drugs used in children are untested in pediatric populations Young children cannot evaluate or express their own response to medications Pediatric dosage forms not available Children metabolize and transport drugs differently than adults