* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download SEMINAR ON - Pharmawiki.in
Compounding wikipedia , lookup
Orphan drug wikipedia , lookup
Drug design wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Nicholas A. Peppas wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
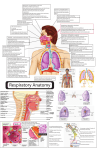
BY V. SANDEEP KUMAR M.PHARMACY II ASEMESTER 2010 DEPARTMENT OF PHARMACEUTICS, UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES, KAKATIYA UNIVERSITY, WARANGAL. Clinical testing of IN Morphine gluconate compared with traditional IM and oral products CONTENTS INTRODUCTION ANATOMY AND PHYSIOLOGY OF NASAL CAVITY BARRIERS TO NASAL ABSORPTION FACTORS INFLUENCING NASAL DRUG ABSORPTION STRATEGIES TO INCREASE NASAL DRUG ABSORPTION NOSE TO BRAIN DELIVERY INTRANASAL DELIVERY OF VACCINES INTRANASAL DELIVERY OF PEPTIDE AND PROTEINE DRUGS ANIMAL MODELS FOR NASAL ABSORPTION STUDIES THERAPEUTIC AREAS SUTIABLE FOR INTRANASAL DELIVERY CONCLUSION REFERENCES Avoidance of hepatic first-pass metabolism Avoids degradation of drug in gastrointestinal tract resulting from acidic or enzymatic degradation Rate of absorption comparable to IV medication Results in rapid absorption and onset of effect Non-invasive, Painless, needle-free administration mode Easily accessible (even easier to access than IM or IV sites) Self-medication is possible through this route Results in higher bioavailability thus uses lower dose & hence lower side effects Useful for both local & systemic drug delivery Direct transport into systemic circulation and CNS is possible . Offers lower risk of overdose Drugs that are orally not absorbed can be delivered to the systemic circulation by nasal drug delivery Adversely affected by pathological conditions(cold or allergies may alter significantly the nasal bioavailability) Irritation of nasal mucosa by drugs Volume that can be delivered into nasal cavity is restricted to 25–200 μl Normal defence mechanisms like mucocillary clearance and ciliary beating affects the permeability of drug Enzymatic barrier to permeability of drugs Interspecies variability is observed in this route Absorption enhancers cause irritation. NASAL CAVITY :ANATOMY, PHYSIOLOGY Major functions of the nasal cavity are breathing and olfaction. Nasal vasculature is richly supplied with blood to fulfill the basic functions such as heating and humidification, mucociliary clearance and immunological functions. Relatively large surface area (~150 cm2) because of the presence of ~400 microvilli per cell. It is divided by middle (or nasal) septum into two symmetrical halves, each one opening at the face through nostrils and extending posterior to the nasopharynx. Cross-sectional View a – nasal vestibule d – middle turbinate b – palate e – superior turbinate c – inferior turbinate f – nasopharynx Nasal secretions Nasal secretion contains sodium, potassium, calcium, mucus glycoproteins, albumins, immunoglobulins IgA, IgG, lysozymes, cytochrome P450 dependent monooxygenases, lactate dehydrogenase, oxidoreductases, hydrolases like steroid hydrolases Nasal pH It varies between 5.5–6.5 in adults and 5.0–7.0 in infants. Nasal epithelium is covered with a thin mucus layer (5 μm thick) and organized in two distinct layers: an external, viscous and dense(gel), and an internal, fluid and serous(watery). Nasal mucus layer consists of 95% of water, 2.5-3% of mucin, and 2% of electrolytes, proteins, lipids, enzymes, antibodies, sloughed epithelial cells and bacterial products MUCOCILIARY CLEARANCE(MCC) Nasal mucosal lining Enzymes present in nasal cavity Mucociliary clearance (MCC) •Lipophilic drugs are generally well absorbed with the pharmacokinetic profiles identical to those obtained after an I.V injection and bioavailabilities approaching 100%. •Ex: fentanyl where the Tmax for both i.v and nasal administration is 7 min or less and the bioavailability was near to 80%. • Nasal permeability of polar drugs especially large mol.wt polar drugs such as peptides and proteins is low. •Polar drugs with mol.wt below 1000 Da will generally pass the membrane using paracellular route. Tight junctions can open and close to a certain degree, when needed. Proteins through endocytotic transport process but only in low amounts. Clearance of the administered formulation from the nasal cavity due to the mucociliary clearance mechanism. Especially for drugs that are not easily absorbed and formulations that are not mucoadhesive. Aldehyde dehydrogenase, glutathione transferase, epoxide hydrolases, cytochrome P-450-dependent monooxygenases, carboxyl esterases ex: nasal decongestants, alcohols, nicotine and cocaine. Aminopeptidases, exopeptidases, endopeptidases are involved in in pre systemic degradation of peptides and proteins Transport Of Drugs Across Nasal Epithelium A- Transcellular passive diffusion, B- Paracellular passive diffusion, C-Carrier mediated , D- Transcytosis , E- Effluxt ransport NASAL PHYSIOLOGICAL FACTORS Blood flow and neuronal regulation Huang et al showed that phenylephrine, a vasoconstrictor agent, inhibited the absorption of acetylsalicylic acid in nasal cavity. Kao et al. stated that nasal absorption of dopamine was relatively slow and incomplete probably due to its own vasoconstrictor effect. Nasal secretions • Viscosity of nasal secretion • Diurnal variation pH of nasal cavity Mucociliary clearance (MCC) The clearance of a drug product from the nasal cavity is influenced by the site of deposition. Polar drugs are the most affected by MCC. Inter-individual variability observed in MCC. Enzymatic degradation Transporters and efflux systems Physicochemical properties of drugs Molecular weight Lipophilicity pKa Lipophilic drugs well absorbed through transcellular mechanisms with nasal bioavailability near to 100%( lower than 1 kDa). Absorption of lipophilic drugs bigger than 1 kDa is significantly reduced. Rate of permeation of polar drugs is highly sensitive to mol.wt if it is higher than 300 Da. For some small polar molecules only a 10% bioavailability is suggested. The value may go down to 1% for large molecules such as proteins. Huang, C.H. et al. studied absorption of benzoic acid at pH 7.19 (99.9% of the drug existed in ionized form) it was found that >10% of drug was absorbed. Solubility Drugs poorly soluble in water and/or requiring high doses may constitute a problem as allowable volume of drug solution is low for intranasal drug administration PROPERTIES OF THE FORMULATION pH Viscosity Osmolarity Pharmaceutical excipients Area of nasal mucus membrane exposed Dosage form Device related factor Particle size of the droplet or powder If the particle size is <10 μm, then particles will be deposited in the upper respiratory tract, whereas if particle size is <0.5 μm then it will be exhaled. Size between 5–7 μm will be retained in the nasal cavity. Site and pattern of deposition STRATEGIES TO INCREASE NASAL DRUG ABSORPTION Prodrugs To improve the solubility of poorly soluble drugs. Ex: the l-dopa has a low water solubility of 1.65 mg/Ml, Testosterone, Estradiol Kao et al. produced various prodrugs of L-Dopa and the solubility was increased to 660 mg/mL with butyl ester prodrug. To improve its lipophilic character, ultimately increasing its transport across a biological membrane To improve enzymatic stability of drugs. For example, Yang et al. stated that L-aspartate- β-ester prodrug of acyclovir was more permeable and less labile to enzymatic hydrolysis than its parent drug. It is a a powerful strategy to increase the bioavailability of peptides. Choice of salt form Cancer patients treated with nasally administered morphine gluconate experienced rapid onset of pain relief and good pain scores (Fitzgibbon et al., 2003; Pavis et al., 2002). Co-solvents Diazepam and Clonazepam are administered to suppress epileptic convulsions requires rapid onset of action. However, these are poorly soluble and nasal formulations comprised of cosolvents demonstrated a Tmax of <5 min, and a pharmacodynamic response was seen in 1.5 min in a rabbit model (Li et al.,2000) Enzymatic inhibitors Proteases and Peptidases inhibitors - bestatine, amastatin, boroleucin, borovalin, and comostate amylase, puromycin, bacitracin (Ex: leucine enkephalin and human growth hormone) Trypsine inhibitors – leupeptine and aprotinin (against degradation of calcitonin). Certain absorption enhancers - bile salts and fusidic acid. Absorption enhancers They improve the absorption of poorly permeable molecues across nasal epithelium. Physicochemical effects By alterig the physicochemical properties of a drug in the formulation. Membrane effects Induce reversible modifications of the structure of epithelial barrier. oModifying the phospholipidic bilayer, oIncreasing membrane fluidity by a) Extraction or leaching of membrane components ( proteins) b) Creating disorders in the phospholipids domain in the membrane. oReversed micelle formation between membranes. oOpening tight junctions between epithelial cells. Nasal Absorption Promoting Systems Chitosan Due to its biodegradability, biocompatibility and bioadhesive property, lower toxicity, it is widely used in intranasal formulations. It interacts with protein kinase C system and opens the tight junctions between epithelial cells. It also enhances the dissolution rate of low water soluble drugs. Most studied drugs are insulin and morphine Cyclodextrins As complexing agents to improve nasal drug absorption by increasing drug solubility and stability. (2-hydroxypropyl-cyclodextrin increased the solubility of progesterone 88-fold.) They interact with the lipophilic components of membranes changing their permeability. A nasal product (Aerodiol) containing 17-estradiol solubilized in dimethylcyclodextrin is marketed for menopausal symptoms. Mucoadhesive drug delivery systems Mucoadhesion implies the attachment of the drug delivery system to the mucus, involving an interaction between mucin and a synthetic or natural polymer is called mucoadhesive. Mucoadhesives mostly used in IN delivery are chitosan, alginate and cellulose or its derivatives. Carbapol 934P and polycarbophil are mucoadhesive polymers that inhibit the trypsin proteolytic enzyme and therefore, increase the stability of peptide drugs. NOVEL DRUG FORMULATIONS Liposomes They can effectively encapsulate small and large molecules with a wide range of hydrophilicity and pKa values . They enhance nasal absorption of peptides such as insulin and calcitonin by increasing their membrane penetration (attributed to the increasing nasal retention of peptides, protection of the entrapped peptides from enzymatic degradation ). Novel mucoadhesive multivesicular liposomes for transmucosal insulin delivery has been investigating. Lliposomal drug delivery systems were also reported as useful for influenza vaccine and non-peptide drugs such as nifedipine. Microspheres Microspheres based on mucoadhesive polymers (chitosan, alginate) present advantages for IN delivery. Microspheres may also protect the drug from enzymatic metabolism. Wang et al. have investigated gelatin microspheres as a IN delivery system for insulin . Positive results are found for nasal delivery of Metoclopramide microspheres of alginate/chitosan Carbamazepine chitosan microspheres Carvedilol alginate microspheres New and developing approach to deliver drugs to the brain. Improved delivery to the brain via the IN route has been reported for some low-mol.wt drugs as well as therapeutic peptides and proteins . Nose to brain delivery has been reported either in humans or animal models of Alzheimer’s disease, brain tumours, epilepsy, pain and sleep disorders . Nose to the CNS may occur via olfactory neuroepithelium. Since central nervous bioavailability of drugs, transported by the olfactorypathway is estimated to be 0.01% to 0.1%, only very potent drugs may reach therapeutic levels Possible routes of transport between the nasal cavity and the brain and CSF Efflux transporters impair drug concentration in the brain after IN administration [Graff and Pollack 2003]. P-glycoprotein, an ATP-dependent efflux pump, preventing the influx of a drug (D) from nasal membrane to CNS Human clinical testing of IN Apomorphin, Sublingual and subcutaneous dosing in 12 subjects INTRANASAL DELIVERY OF VACCINES Nasal mucosa houses lymphatic tissues involved in the first line defense against airborne microorganism. In humans the NALT is known as the Waldeyer´s Ring. Reasons for exploiting the nasal route for vaccine delivery. • The nasal mucosa is the first site of contact with inhaled pathogens. • The nasal passages are rich in lymphoid tissue. • Creation of both mucosal and systemic immune responses. • Low cost, patient friendly, non-injectable, safe. The majority of the invading pathogens enter the body via mucosal surfaces. Therefore, mucosal sites have a potential as first line of defense against entering pathogens. Nasal secretions are known to contain immunoglobulins (IgA, IgG, IgM, IgE), and neutrophils and lymphocytes in the mucosa . Nasal vaccine delivery stimulates the production of local secretory IgA and IgG Nasal vaccine systems based on live or attenuated whole cells, split cells, proteins or polysaccharides and with and without various adjuvants were investigating. Nasal Drug Products for Vaccination Available in the Market The chitosan nasal delivery system has been tested for influenza, and diphtheria vaccine in various animal models and in man. Bioadhesive property and transient effect on the tight junctions of chitosan lead to an improved immune response. It has been reported that Ab levels were similar for IM conventional influenza vaccine and nasal administration of the chitosan-influenza vaccine. Due to its positive charge chitosan gets complexed with negatively charged DNA plasmids and self-assembling into nanoparticulate systems for improved delivery of DNA. Nasal delivery of DNA plasmid expressing epitopes of respiratory syncytial virus (RSV) to produce an effective vaccine. INTRANASAL DELIVERY OF PEPTIDE AND PROTEIN DRUGS •Being hydrophilic polar molecules of relatively high molecular weight, are poorly absorbed across biological membranes with low bioavailabilities . •This low uptake may adequate for some commercial products such as desmopressin and calcitonin ( 3432 Da, 3% (Novartis Pharmaceuticals, 2006). •Novel formulation strategies Absorption enhancers Bioadhesive agents •Absorption enhancing effect of different cyclo-dextrins (rats,rabbits), medium chain fatty acid (rats), sodium tauro-24, 25-dihydrofusidate (sheep) on intranasally administered insulin in rats and rabbits was obsrved. Bioavailabilities of peptides and proteins administered IN in the presence of absorption enhancers The clearance half- life can be increased with starch microspheres of insulin (SMS). Insulin administered in combination with SMS resulted in 497% increase in AUC for plasma insulin as compared to insulin solution. The AUC increased by 1657% compared to insulin solution when an enhancer lysophosphatidyl choline was used with insulin and SMS. Successfully intranasally administered proteins include oxytocin, buserelin, desmopressin, luteinizing hormone releasing hormone, growth hormone and adrenocorticotrophic hormone. Nasal Drug Products (Proteins and Peptides) for Systemic Drug Delivery on the Market Nasal Drug Products (Non-Peptide) for Systemic Drug Delivery on the Market In Vivo Nasal Absorption studies Rat Model Rabbit Model Dog Model Sheep Model Monkey Model Ex Vivo Nasal Perfusion Models The surgical procedure of Nasal absorption in the rat Experimental set-up for exvivo Nasal perfusion Nasal Dosage forms Nasal Drops Simple and convenient systems . Disadvantage is the lack of dose precision. Nasal Sprays Both solution and suspension can be formulated into nasal sprays. They can deliver an exact dose of 25 to 200 µl by metered dose pumps and actuators. The choice of pump and actuator assembly depend on the particle size and morphology (for suspensions) and viscosity of the formulation. Solution and suspension sprays are preferred over powder sprays because powder results in mucosal irritation . Nasal Gels Nasal gels are high-viscosity thickened solutions or suspensions. Advantages Reduction of post-nasal drip due to high viscosity Reduction of taste impact due to reduced swallowing Reduction of anterior leakage of the formulation Reduction of irritation by using soothing/emollient excipients Nasal Powders If solution and suspension dosage forms cannot be developed e.g.due to lack of drug stability Advantages Absence of preservative Superior stability Local application Diadvantages Nasal mucosa irritationa, metered dose delivery NASAL DELIVERY DEVICES Common devices are Droppers Squeeze bottles Spray pumps / atomizers (Accuspray Nasal Atomizer, MAD (Mucosal Atomization Device, nasal) Gel applicators Nasal Nebulisers (Sinus Nebuliser Rhino Clear) Pressurised Metered Dose Inhalers (pMDIs) Nasal (Ex: Landmark®) Disposable Unit/Bi-dose dispensing devices Powder Dispensing Systems MAD Mechanical Basic Pump The unit-dose and the bi-dose system Powder unit-dose system. Powder bi-dose system. Novel Nasal spray pumps Patient-independent Pumps To minimise dose and spray variations related to the patient's hand actuation mode. (Equdel by Valois Pharma) Preservative Free Systems (PFS) To accommodate preservative-free drug formulations. Preservatives may induce itching in chronic use Can generate some formulation instabilities Affect the smell and/or taste of the drug product. (Freepod by Valois Pharma) Side-actuation Spray Pumps Eliminate any risk of the nasal nozzle entering the nostril too deeply Avoids contact between fingers and nostrils which improves hygiene during treatment. LEADING PUMP SUPPLIERS Valois, France Pfeiffer, Germany Becton Dickinson, France Nemo, Spain BREATH ACTUATED BIDIRECTIONAL NASAL DRUG DELIVERY Developed by OptiNose Based on two nasal anatomical features First, during exhalation against a resistance the soft palate closes, separating the nasal and oral cavities. So small particles in nasal spray can be used and still avoid lung deposition by exhaling through the mouth during nasal administration. Single-use bi-directional delivery device Second, during closure of the soft palate there is a communication pathway between the two nostrils, located behind the nasal septum. It is possible for air to enter via one nostril, turn through 180˚ passing through the communication pathway, and leave by the other nostril. Bidirectional Nasal Drug Delivery Principle Comparing Deposition patterns of traditional spray pump and bidirectional delivery device incorporating the same spray pump (Gamma scintigraphy images from the same subject; Cumulative distribution during 32 minutes) White areas in the nose: 20-100% of maximum intensity Orange areas in the nose: 0-20% of maximum intensity Green areas in the nose: no deposition Gamma-scintigraphy images from the same subject; Cumulative distribution during 32 minutes THERAPEUTIC AREAS SUTIABLE FOR INTRANASAL DELIVERY CONCLUSION In a nut shell, the advantages of IN delivery are numerous and very importantly it is rapid and non-invasive. An alternative to parenteral and oral route. IN delivery suitable for both topical or systemic delivery and to treat both acute and chronic diseases. It also bypasses the BBB and delivers the drug directly into the CNS. It reduces systemic exposure and thus reduces the side effects. IN delivery can be utilized for high molecular- weight drugs such as peptides and proteins, however, bioavailability is dependent upon the presence of absorption enhancers. Another application for IN dosing is vaccine therapeutics. However, still some research needs to be conducted in delivery of peptide and protein and vaccines through nasal delivery and delivery of drug from nose to brain. Further, extensive research at the molecular level is required to increase the permeation of drugs through the nasal mucosa without compromising normal function. Taking into consideration the current research interest in nasal delivery and positive outcomes from the clinical trials throughout the world it is expected a wide range of nasal products reaching the market in the near future. REFERENCES • Costantino HR, Illum L, Brandt G, Johnson PH, Quay SC. Intranasal delivery: Physicochemical and therapeutic aspects. Int J Pharm, 2007; 337:1-24. • Chien YW, Su KSE, Chang .S,Nasal systemic drug deliver, 2nd edition, USA:Marcel Dekker;1989.1-38,89-97. • Krishnamoorthy R, Mitra AK. Prodrugs for nasal delivery. Adv Drug Deliv Rev 1998; 29: 135 146. • Talegaonkar S, Mishra PR. Intranasal delivery: An approach to bypass the blood brain barrier. Indian J Pharmacol 2004; 36(3): 140-147 • Arora P, Sharma S, Garg S. Permeability issues in nasal drug delivery. Drug Discov Today 2002; 7(18): 967975 • Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci 2000; 11: 1-18 • Y.W. Chien, S.F. Chang, Intranasal drug delivery for systemic medication, Crit. Rev. Ther. Drug Carrier Syst. 4 (1987) 67 • S. Hirai, T. Yashiki, T. Matsuzawa, H. Mima, Absorption of drugs from the nasal mucosa of rat, Int. J. Pharm. 7 (1981) 317–325. • H.D. Kao, Enhancement of delivery of L-Dopa by the administration of it’s prodrugs via the nasal route, University of Kentucky, Lexington, Kentucky, 1995. • .N. Geurkink, Nasal anatomy, physiology, and function, J Allergy Clin. Immunol. 72 (1983) 123 128. • Illum L. Nasal drug delivery: possibilities, problems and solutions. J Control Release, 2003; 87:187-198. • Illum L. Nasal drug delivery: new developments and strategies. Drug Discov Today, 2002; 7:1184-1189. • Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci, 2000; 11:1-18. http://www.ehow.com/nasal-spray http://www.valoispharma.com http://www.pfeiffer-group.com