* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download + [O] - MrFisherChemistry
Survey
Document related concepts
Transcript
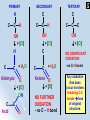
AS Chemistry OXIDATION OF ALCOHOLS OXIDATION OF ALCOHOLS Oxidised to aldehydes and acids or ketones NOT SIGNIFICANT for TERTIARY alcohols! for PRIMARY alcohols -CH2OH for SECONDARY alcohols -C-CH(OH)-C- NB Oxidant = hot acidified Cr2O72- [dichromate(VI)] ion] Provided by mixture of potassium dichromate(VI), K2Cr2O7, and excess dilute sulphuric acid, H2SO4. Oxidant is represented by : [O] Orange Cr2O72- reduced to green Cr3+ PRIMARY SECONDARY C H C C C C H OH + [O] H C + H2O Aldehyde O + [O] OH C Acid C H C OH + [O] C TERTIARY C O C C C C C OH + [O] NO SIGNIFICANT OXIDATION + H2O Ketone O + [O] NO FURTHER OXIDATION - no C – H bond - no C–H bond Any oxidation that does occur involves breaking C-C bonds loss of original structure. What are the oxidation products of : 1. Ethanol? 2. Methyl-propan-2-ol? 3. Butan-2-ol ? CH3CH2OH CH3C(CH3)(OH)CH3 Primary Tertiary Secondary 1. CH3CH2OH + [O] CH3CHO + H2O CH3CHO + [O] CH3COOH CH3CH2CH(OH)CH3 Ethanal Ethanoic acid 2. No significant oxidation 3. CH3CH2CH(OH)CH3 + [O] CH3CH2COCH3 + H2O Butanone Controlling Oxidation Products To Maximise the Production of an ALDEHYDE To Maximise the Production of an ACID or a KETONE This is PARTIAL oxidation This is FURTHER oxidation Use only molar proportions of dichromate and acid Use excess of dichromate and acid Use minimum contact time between alcohol and oxidant by use of immediate distillation Use maximum contact time between alcohol and oxidant by use of reflux Add acidified dichromate to the alcohol to avoid the presence of excess oxidant Pre-mix acidified dichromate and alcohol to ensure excess oxidant at all times Click here for Distillation and Reflux Apparatus Click on the options to select the possible oxidation products (MAY BE MORE THAN ONE) derived from : Methanal Methanoic acid Methanone None Propanal Propanoic acid Propanone None Propanal Propanoic acid Propanone None Pentan-3-al Pentan-3-oic acid Pentan-3-one None 2-Methylbutanal 2-Methylbutanoic acid None Methanol Propan-2-ol Propan-1-ol Pentan-3-ol 2-Methylbutan-2-ol 2-Methylbutanone The End Distillation Reflux