* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Survey
Document related concepts
Transcript
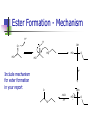
Experimental Reports Next week is the final week of practicals Make sure you are up to date with your reports by next week – all reports Final reports must be handed up no later than one week after completion of final practical – otherwise marks will be forfeited Experiment 17 Reactions of Alcohols and Carboxylic Acids Introduction Given samples of various alcohols and carboxylic acids Establish some of their physical and chemical properties & perform various reactions HO HO ethanol HO pentanol benzyl alcohol O HO O ethanoic acid OH propanoic acid Introduction You will perform solubility and acidity tests on all the compounds provided Ester formation from an alcohol and carboxylic acid Oxidation of an alcohol to a carboxylic acid using permanganate solution Solubility Tests To 1ml of water in three test tubes, add each of the alcohols dropwise until they cease to dissolve or until 10 drops have been added Record your observations Repeat with the two carboxylic acids Comment on your conclusions regarding the solubilities in water Acidity Tests Use the aqueous solutions of ethanol and ethanoic acid from the solubility tests Test both with blue litmus paper Add 1ml of 10% NaHCO3 to each Record your observations in each case Acidity Tests Dissolve 0.5g of sodium acetate in 5ml water – note if there is any odour Add 1ml dilute H2SO4 and again note if there is any odour Record your observations in each test and explain any reactions observed Ester Formation Already encountered in Experiment 14 Heat 2ml of ethanol, 1ml ethanoic acid and a few drops of conc H2SO4 for 5 mins Note any changes you observe – odour? O O OH ethanol + H+ HO ethanoic acid O ethyl acetate Ester Formation - Mechanism H+ H O OH O HO HO Include mechanism for ester formation in your report HO O HO H+ OH2 O -H2O O -H+ HO O Oxidation of Phenylmethanol Phenylmethanol (benzyl alcohol) can be oxidized to give benzoic acid To 1ml of phenylmethanol in a conical flask add 25ml of KMnO4 solution and 0.5g of sodium carbonate Heat on the water bath for 20 mins Cool & acidify with conc HCl Oxidation of Phenylmethanol Add sodium sulphite solution until the brown maganese dioxide dissolves Cool to room temperature – white precipitate of benzoic acid settles out HO HO KMnO4 O benzyl alcohol benzoic acid Oxidation of Phenylmethanol Isolate crystals on the Hirsch funnel, wash with water & dry Get melting point when dry Calculate % yield from number of moles of phenylmethanol you began with Calculations Density of phenylmethanol = 1.05 gml-1 Use the density & volume to calculate the mass of phenylmethanol used m V m .V m n MR Calculations Use the mass of phenylmethanol to calculate the number of moles When dry, weigh the benzoic acid product and thus calculate the number of moles of product % yield = moles benzoic acid/moles phenylmethanol Report Introduction All observations from the solubility and acidity tests & reactions Mechanism for ester formation Weight and melting point % yield of benzoic acid Discussion & conclusions