* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Colorimeters
Dispersion staining wikipedia , lookup
Surface plasmon resonance microscopy wikipedia , lookup
Cross section (physics) wikipedia , lookup
Optical coherence tomography wikipedia , lookup
Atomic absorption spectroscopy wikipedia , lookup
Harold Hopkins (physicist) wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Retroreflector wikipedia , lookup
Johan Sebastiaan Ploem wikipedia , lookup
Thomas Young (scientist) wikipedia , lookup
Atmospheric optics wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Opto-isolator wikipedia , lookup
Photographic film wikipedia , lookup
Transparency and translucency wikipedia , lookup
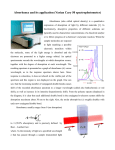
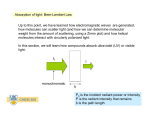
Colorimeters Definition colorimeter is an instrument which compares the amount of light getting through a solution with the amount which can get through a sample of pure solvent. A Substances absorb light for a variety of reasons. Pigments absorb light at different wavelengths. A cloudy solution will simply scatter/block the passage of light (sometimes a colorimeter is used to monitor the growth of a bacterial or yeast culture). The % transmission or the % absorbance is recorded (you can use either). It is possible to change the color of the light that is used by using filters in the simplest equipment or an "optical wedge" . How the Colorimeter Works Light from a LED light source passes through a Cuvette containing a solution sample, Some of the incoming light is absorbed by the solution. As a result, light of a lower intensity strikes a photodiode. Construction The essential parts of a colorimeter are: a light source, which is usually an ordinary filament lamp an aperture which can be adjusted a detector which measures the light which has passed through the solution a set of filters in different colors filters are used to select the wavelength of light which the solution absorbs the most. Solutions are usually placed in glass or plastic cuvettes. (1) (2) (3) (4) (5) (6) (7) (8) Wavelength selection, Printer button Concentration factor adjustment, UV mode selector (Deuterium lamp) Readout Sample compartment Zero control (100% T), Sensitivity switch. TRANSMITTANCE Transmittance “T” The amount of light that passes through a solution is known as transmittance T. Transmittance can be expressed as the ratio of the intensity of the transmitted light It to the initial intensity of the light beam Io The transmittance formula is: T = It /Io The Colorimeter produces an output voltage which varies in a linear way with transmittance, allowing a computer, calculator, or handheld to monitor transmittance data for a solution. Absorbance “A” The reciprocal of transmittance of the sample varies logarithmically (base ten) with the product of three factors: ε, the molar absorptivity of the solution, b the cell or cuvette width, and C the molar concentration A = log(1/T) = ε b C The relationship between these two variables is inverse and logarithmic (base 10). It can be expressed as A = log(1/T) T&A T&A RELATION BETWEEN ABSORBANCE AND CONCENTRATION BEER’S LAW Beer’s law Mathematical statement of Beer’s law For a given solution contained in a cuvette with a constant cell width, the Absorbance is proportional to the concentration: A=εC b=kC This equation shows absorbance to be related directly to concentration and represents a mathematical statement of Beer’s law. b is cuvette path length C is concentration of absorbing substance ε is absorptivity (~ substance) In many chemical and biological experiments this law is assumed for making calibration line for determining concentration. Beer’s Law Curve Determination of C of an unknown substance To obtain a Beer’s law curve, several standards (solutions of known concentration) are prepared and their absorbance values are determined using a Colorimeter. A graph of absorbance vs. concentration is then plotted. A solution of unknown concentration is placed in the colorimeter and its absorbance measured. When the absorbance of this solution is interpolated on the Beer’s law curve, its concentration is determined on the horizontal axis. Alternatively, its concentration may be found using the slope of the Beer’s law curve. Concentration of unknown solution Cu Cu= Cs Au/As Cu is unknown concentration Au is unknown absorbance Cs standard concentration As is standard absorbance Ranges of A and T for the colorimeter during calibration For best results our laboratory testing of the colorimeter indicates that absorbance or transmittance values should fall with these ranges: Transmittance (T) 0.28 - 0.90 Absorbance (A) 0.050 - 0.550 To get sufficiently reliable results calibration solutions should be used which lie in the flat part of the curve dT% = 1% (i.e. 1% accuracy of the transmission). The measurement error in percent in the concentration (or absorbance) dc/c as function of the transmission T for different accuracy's (dT%) of the transmittance range. Determining of the wavelength You can select three LED light colors: red (635 nm), green (565 nm) or blue (470 nm). There are several ways you can decide which of three wavelengths to use: Method 1. Look at the color of the solution. (Remember that the color of solution is the color of light which is not absorbed). use a different color of light that will be absorbed For example: with a blue CuSO4 solution, use the red LED (635 nm). Method 2. Place a cuvette containing the solution in the colorimeter. And check to see which of three wavelengths yields the highest absorbance (low transmittance). Method 3. Directions for most colorimetry experiments express a recommended wavelength. Use the closest of the three wavelengths on the colorimeter. Even if the LED wavelength is somewhat different, a Beer's law curve can usually be obtained at almost any wavelength around the recommended wavelength. Basic Colorimeter Schematic Electronics Calibration Example Calculations Vo Vo Vo Vo Automatic CAL ….each time before use After powering up the Colorimeter and waiting for a 5 minute warm up, press to select a wavelength. Open the lid, insert a cuvette filled about 3/4 full of distilled water, and close the lid. Make sure that one clear side of the cuvette is lined up with the arrow at the top of the cuvette slot. Press the CAL button and hold it until the red LED begins to flash and then release the CAL button. When the LED stops flashing, the calibration is complete. Detector Silicon Photo-diode Filter options Interchangeable glass or interference type filter 420 nm, 470 nm, 510 nm, 530 nm, 620nm, 600 nm Light source Tungsten lamp 3.0 V 0.5 A Display LCD Cuvette Round 10 mm ID, 12 mm OD 105mm L Square 10 mm x 10 mm x 45 mm Wavelength range 420 nm - 660 nm Photometric Range Transmittance 10 ~ 100% Absorbance 0.01 - 1 ABS Power NiCd battery or line adapter Dimensions 150 mm x 80 mm x 50 mm Weight ~ 1 lb Specifications for the PC-10 handy photo colorimeter Maintenance Calibration Adjustment Replacement of burned-out lamp and photo detector Electronic problems are rare